02 – PREPARATION AND CHARACTERIZATION OF Sr-CELSIAN DERIVED FROM A ZEOLITE PRECURSOR SYNTHESIZED FROM AMAZONIAN BAUXITE RESIDUE
Ano 12 (2025) – Número 3 – Mineral Synthesis Artigos

10.31419/ISSN.2594-942X.v122025i3a2NC
Nayara Aparecida Fonseca Couto1
Bruno Apolo Miranda Figueira1*
Helcio José dos Prazeres Filho2
Marcondes Lima da Costa3
1Postgraduate Program in Materials Engineering, Federal Institute of Education, Science and Technology of Pará, Brazil
2HYDRO, Paragominas, Pará, Brazil
3Geosciences Institute, Federal University of Pará, Belém-Pará, Brazil.
*Corresponding author: bfigueira@ufpa.br
Received on december 15, 2024; revised and accepted on february 2, 2025.
ABSTRACT
This study explores the potential of bauxite waste from the Amazon in Brazil for producing Sr-celsian ceramics. The X-ray diffraction (XRD) analysis revealed the waste to be composed of gibbsite, kaolinite and hematite as main mineral, and anatase and quartz as accessories. This residue composition by addition of NaOH can be transformed into hydroxysodalite zeolite, followed by its transformation into Sr-celsian ceramic phases by addition of Sr2+. Thermal conversion of strontium zeolite to Sr-celsian was achieved as low as 800°C. However, XRD analysis also detected nepheline, indicating incomplete conversion. This suggests the need for further optimization to achieve complete transformation into Sr-celsian. Future investigations should focus on understanding factors promoting nepheline formation during the conversion process. Overall, this study highlights the potential of utilizing bauxite waste for valuable Sr-celsian ceramics, with further optimization required to enhance conversion efficiency.
Keywords: Strontium aluminosilicate, feldspar, hydroxysodalite, ceramic.
INTRODUCTION
Strontium feldspar, like a Sr-celsian or celsian ceramic, possesses exceptional properties such as high melting temperature, low thermal expansion coefficient, high mechanical strength, high chemical stability, and advantageous dielectric characteristics, including low dielectric constant (Ferone et al., 2010; MCCauley, 2000; Liguori et al., 2008; Biesuz et al., 2017). These properties make Sr-celsians a promising choice for various advanced technological applications. Traditional methods for synthesizing this celsian ceramic are complex and time-consuming, requiring over 20 hours at temperatures above 1500°C, resulting in high costs and unsatisfactory yields (Ferone et al., 2010; Biesuz et al., 2017). Other routes that transform zeolitic materials (zeolite A and faujasite) into celsian, have gained interest due to the use of lower cost starting materials such as clay and industrial waste (Matsumoto and Goto, 2009).
Zeolites, crystalline microporous aluminosilicates, are commonly synthesized using commercial chemical reagents. However, this practice has limitations in terms of applicability and cost, driving the search for alternative sources of silicon- and aluminum-rich raw materials, such as fly ash, and industrial, agricultural, and mineral waste (HUDCOVÁ et al., 2021). Utilizing these alternative sources can significantly reduce the production cost of zeolites by up to 95% compared to commercial zeolites (Hua et al., 2023).
Prompted by stricter international regulations regarding mineral waste, research efforts have intensified in recent years to promote the reuse, recycling, and recovery of valuable components, minimizing landfill disposal (Lèbre et al., 2017). In this context, bauxite washing residues, a byproduct of bauxite mining in the Amazon region, has emerged as a promising feedstock for developing various high-value-added and technologically interesting products, including zeolites. Moreover, there has recently been an improvement in mineral processing through the dry-filling technique, which prevents the formation of tailings dams (Nascimento et al., 2019; Nascimento et al., 2022; Melo et al., 2019).
In the present work, an attempt was made to obtain Sr-celsian-type material using bauxite washing residues as cheap and abundantly available raw material.
MATERIALS AND METHODS
Materials
The raw material used in this study consisted of bauxite residue from the bauxite mine located in the municipality of Paragominas, in the Brazilian Amazon region. As a mineralizing agent in the synthesis of zeolite, sodium hydroxide (NaOH) pellets (VETEC) were used. For the preparation of ion exchange aqueous solution, strontium chloride (SrCI2.6H2O) (NEON) were used.
Experimental procedures
Hydroxysodalite zeolite synthesis was performed adapting the methodology described by Wang et al. (2012). Bauxite residue was mixed with NaOH pellets in a mass ratio of 1:2 and heated in a muffle furnace at 350 °C for 2 hours for alkali activation. After naturally cooling down, the sintered product was crushed into a powder and dissolved in 50 ml of distilled water, which was left magnetic stirring for 60 min at room temperature. Hydrothermal treatment of the sample was carried out at 100 °C for 24 hours in a sealed vessel. Then, the solid material obtained was separated through filtration, washed with distilled water until it reached pH~7-8 and coded as Nay-Na-sod.
The ion exchange process occurred under magnetic stirring of the sample in 1M strontium chloride (SrCI2.6H2O) solution for 24 hours. After the procedure, the material was washed with abundant distilled water, filtered, and dried in an oven at 110 °C for 24 hours (Nay-Sr-cel sample). The cation exchanged zeolite was subjected to thermal treatments at temperatures ranging from 800 to 1200°C for 1 h. Heating rate was ~10°C/min, while cooling rate was free.
The chemical composition of the materials was determined using a Bruker S2 Ranger X-ray Fluorescence spectrometer, with a Pd tube, using tablets pressed at 20 tons.
The samples were identified by powder X-ray diffractometry, using a D2 PHASER from Bruker (CuKα = 1.5406 Å) 400 W power, with a Bragg-Brentano geometry in continuous mode, scanning speed of 0.25° /min, using a fast LynxEye detector as the detection system. The voltage was 30 kV and 10 mA, respectively.
Fourier transform infrared spectroscopy (FTIR) analyses were prepared using approximately 0.0015g of the sample and 2g of potassium bromide (KBr, Merck). The pellets were placed in steel disc molds with a diameter of 14 mm and pressed with the pressure of Kbar in a Specac 8 manual press. The analyses were performed in a Vertex 70 from Bruker in the spectral range of 400-4000-1.
RESULTS AND DISCUSSIONS
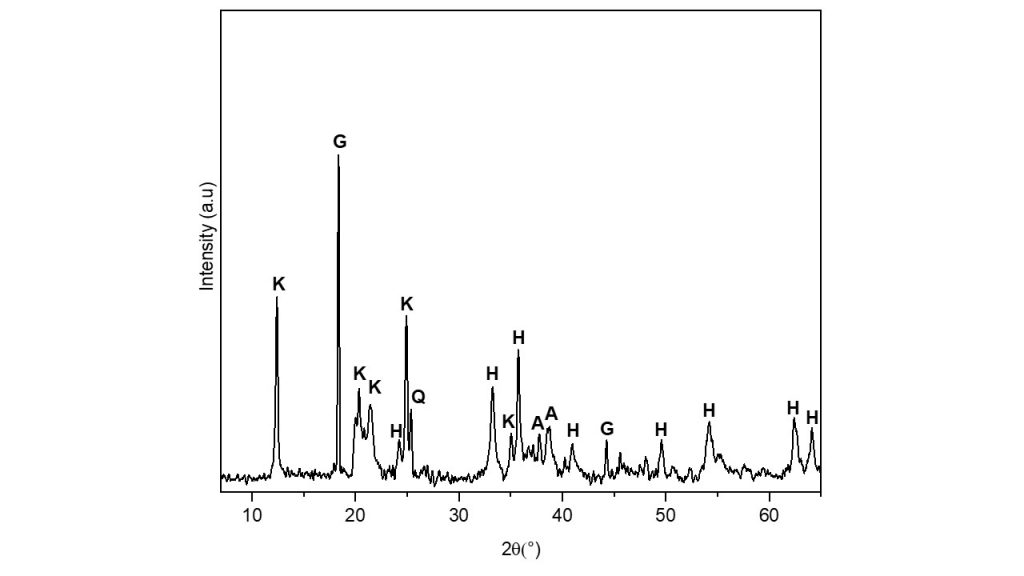
The XRD pattern of bauxite residue shows the presence of gibbsite (Al(OH)3), kaolinite (Al4(Si4O10)(OH)), quartz (SiO2) , hematite (Fe2O3) and anatase (TiO2) (Fig. 1). The chemical composition of the bauxite-residue is listed in Table 1. Significant amounts of aluminum (30.15%), silicon (27.27%) and iron oxides (24.22%) were determined, as well as smaller amounts of titanium oxide (3.93%), which refer to minerals gibbsite, kaolinite, hematite and anatase, respectively.
Figure 1. XRD of bauxite residue sample showing kaolinite (K), gibbsite (G),
hematite (H), quartz (Q) and anatase (A).
Table 1. Chemical composition of the bauxite residue by XRF.
| Composition (%) | Al2O3 | SiO2 | Fe2O3 | TiO2 | Other | LOI | Total |
| Bauxite Residue | 30.15 | 27.27 | 24.22 | 3.93 | 1.29 | 13.14 | 100 |
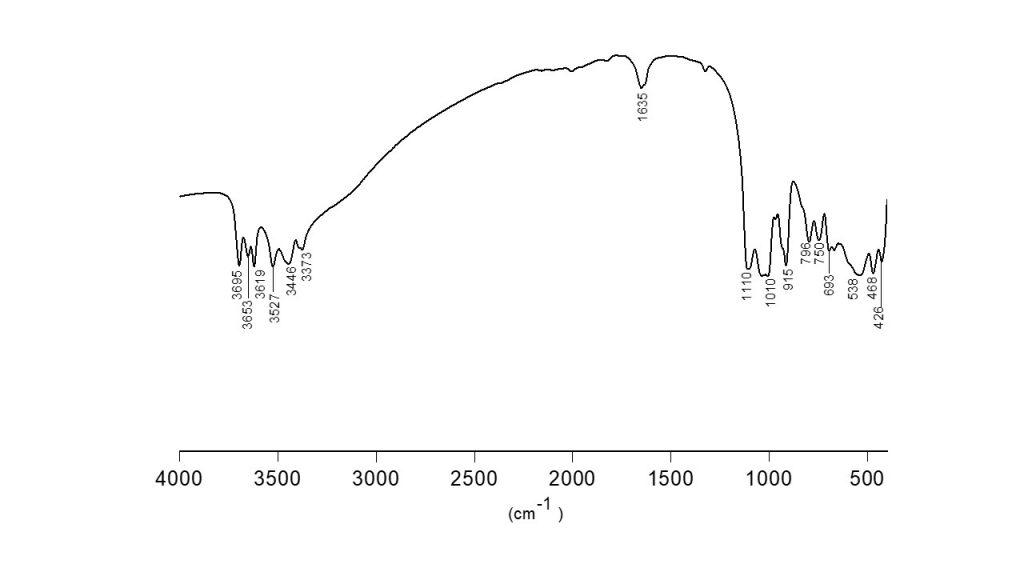
The FTIR spectrum of bauxite residue sample (Fig. 2) shows bands in the regions of 3695, 3653 and 3619 cm-1 that are attributed to the axial deformation of the hydroxyl group of the mineral kaolinite (Bougeard et al., 2000; Balan et al., 2001). The bands displayed in 3527, 3446 and 3376 cm-1 are the OH stretch frequency region referent to gibbsite mineral (Wang et al., 2020). The absorption at 1635 cm-1 is due to water. The bands at 1110 and 1010 cm-1 are related to Si-O stretching modes. The presence of the 915 cm‑1 and 796 cm−1 bands is characteristic of the angular deformation and rotation of the Al – OH bond of the mineral gibbsite (Hoyo et al., 2008). The bands at 538–468 cm–1 is related to the Fe–O functional group for hematite. The band at 426 cm–1 observed in the IR reflection corresponds to the stretching vibrations of the Ti–O bonds of titanium dioxide with the anatase structure (Roudouane et al., 2020; Khorshidi et al., 2017; Mokrushin et al., 2021). This spectroscopic characterization confirmed the presence of crystalline phases in the tailings as previously reported by X-ray diffraction, as well as the absence of amorphous phases of Fe and Al oxyhydroxides, which could also have been present in the starting material.
Figure 2. FTIR spectra of bauxite residue sample.
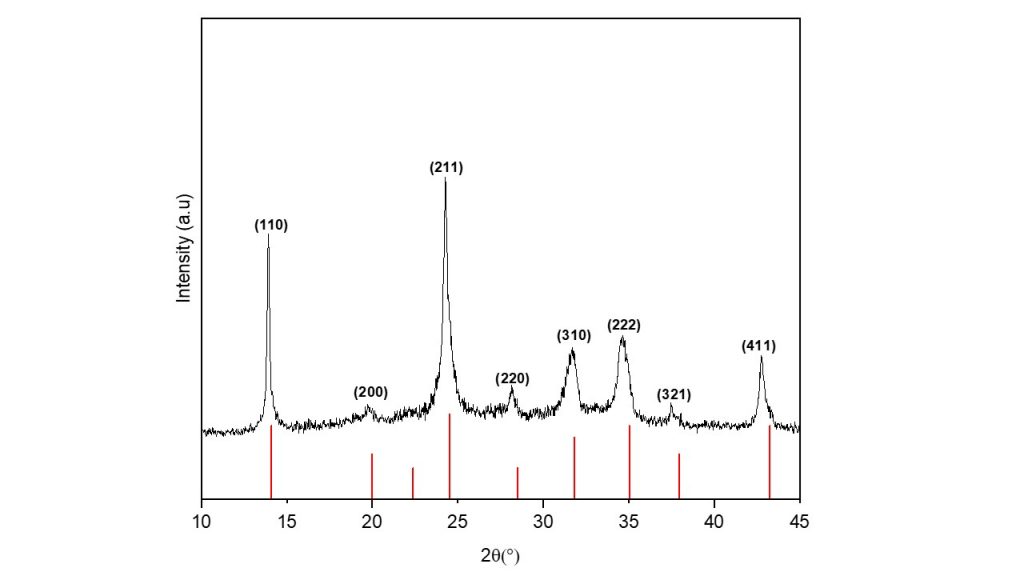
The process to synthesize zeolite from bauxite residue was made at a hydrothermal temperature of 100 °C for 24 h. Fig. 3 gives the XRD pattern of synthetized zeolite (Nay-Na-sod), the diffraction peaks at 13.9°, 19.8°, 24.24°, 28.13°, 31.7°, 34.5°, 37.59° e 42.73° (2 theta), associated to the (110), (200), (211), (220), (310), (222), (321) e (411) planes respectively, corresponded to hydroxysodalite zeolite (ICSD 11-0401).
Figure 3. XRD of hydroxysodalite zeolite synthetized from bauxite residue and NaOH.
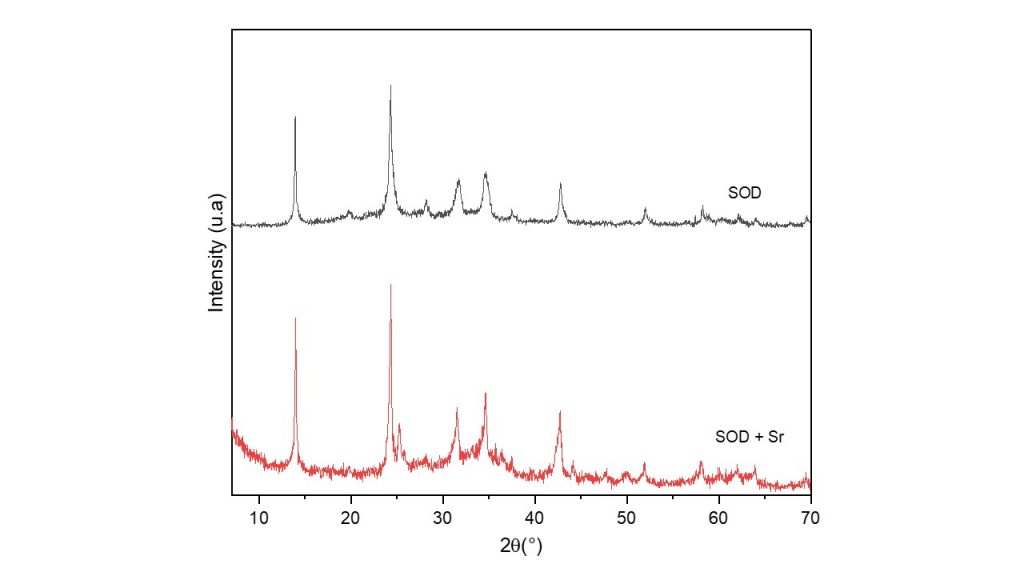
The XRD pattern of the hydroxysodalite zeolite exchanged with Sr cations (Nay-Sr-cel sample) (Figure 4, red line) showed that the zeolitic matrix was maintained after the insertion of the cations and that there was a narrowing and elongation of the peaks at 13.9°, 19.8°, 24.24°, 28.13°, 31.7°, 34.5°, 37.59° and 42.73° (2 theta), as well as the appearance of new low intensity peaks at 25.2, 33.2, 36, 35, 44 and 47° (2 theta).
Fig. 4: XRD of Sr-exchanged zeolite hydroxysodalite (red line, SOD + Sr)
and zeolite hydroxysodalite (black line, SOD).
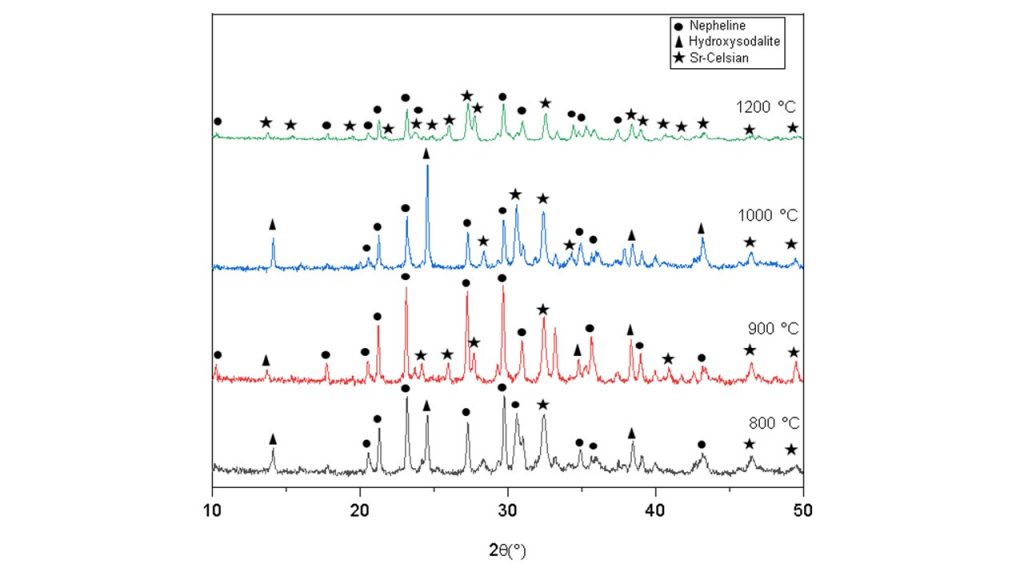
To investigate the crystallization process, Sr-exchanged hydroxysodalite was heated at various temperatures for 1 h. Figure 5 shows the XRD patterns of the obtained products. When Sr-exchanged hydroxysodalite was heated at 800°C, the structure of hydroxysodalite changed and showed peaks characteristic of hydroxysodalite (ICSD 37-0476), nepheline (ICSD 01-083-2376) and a peak characteristic of strontium feldspar at 32.5° (2 theta) (ICSD 38-1454), suggesting the formation of the desired Sr-celsian at higher temperature (800°C/ 1 h). When heated to 900°C, the sample showed an increase in the characteristic peaks of the Sr-celsian phase ICSD 38-1454), indicating that the increase in temperature may favor the conversion of the material into the Srcelsian ceramic phase.
Fig. 5: X-ray diffraction patterns of Sr-exchanged zeolite heated
at various temperatures for 1 h.
In the sample heated to 1000°C, it is possible to observe the persistence of the peaks referring to the sodalite, nepheline and strontium feldspar phases, as well as at the temperature of 1200°C, where the peaks remain with good intensity. These results at distinct temperatures indicate that even at a temperature of 1200 °C for 1 hour, the strontium-doped zeolite did not completely convert into the desired ceramic phase, maintaining secondary phases, being necessary new adjustments in conversion conditions or procedures to achieve full Sr-celsian complete formation.
CONCLUSIONS
The results of this research revealed that the bauxite residue from the Amazon region still consisted of gibbsite (Al(OH)3), kaolinite (Al₄Si₄O₁₀(OH)₈), hematite (Fe₂O₃), anatase (TiO₂), and quartz (SiO₂). The favorable chemical and mineralogical composition of the residue allowed for its conversion into hydroxysodalite zeolite by addition of NaOH, followed by its transformation into Sr-celsian ceramic phases by addition of Cl-chloride. The thermal conversion of strontium zeolite into a ceramic material, Sr-celsian, was investigated at various temperatures (800 °C, 900 °C, 1000 °C, and 1200 °C). This procedure revealed the formation of the Sr-celsian at 800°C and also the formation of nepheline. This observation, alongside the incomplete conversion to Sr-celsian, suggests that adjustments to the conversion conditions or procedures might be necessary. Further investigation is needed to understand the factors promoting nepheline aluminosilicate formation during the conversion process.
Acknowledgements
The authors would like to thank the financial support of the company HYDRO through agreement 4486 – HYDR0/UFPA/FADESP as well as a scholarship for the first author; the author ML Costa thanks the CNPQ for bench rate number 304.967/2022-0.
REFERENCES
BALAN, E.; SAITTA, A. M.; MAURI, F.; CALAS, G. 2001. First-principles modeling of the infrared spectrum of kaolinite, American Mineralogist, vol. 86, pp. 1321-1330. https://doi.org/10.2138/am-2001-11-1201.
BIESUZ, M.; SPIRIDIGLIOZZI, L.; MAROCCO, A.; DELL’AGLI, G.: SGLAVO, V. M.; PANSINI, M. 2017. Sintering behavior of Ba/Sr celsian precursor obtained from zeolite-A by ion-exchange method, Journal of the American Ceramic Society, vol.100, pp. 5433-5443. https://doi.org/10.1111/jace.15117.
BOUGEARD, D.; SMIRNOV, K. S.; GEIDEL, E. 2000. Vibrational Spectra and Structure of Kaolinite: A Computer Simulation Study, The Journal of Physical Chemistry B, vol. 104, pp. 9210-9217. https://doi.org/10.1021/jp0013255.
FERONE, C.; LIGUORI, B.; MARROCO, A.; ANACLERIO, S.; PANSINI, M.; COLELLA, C. 2010. Monoclinic (Ba, Sr)-celsian by thermal treatment of (Ba, Sr)-exchanged zeolite A, Microporous and Mesoporous Materials, vol. 134, pp. 65-71. https://doi.org/10.1016/j.micromeso.2010.05.008.
HOYO, C.; DORADO, C.; RODRÌGUEZ-CRUZ, M. S.; SANCHEZ-MARTIN, M. J. 2008. Physico-chemical study of selected surfactant-clay mineral systems. J Therm Anal Calorim, vol. 94, pp. 227–234. https://doi.org/10.1007/s10973-007-8934-6.
HUA, X.; GAO, Z.; SHI, Y.; HAO, W.; LIU, X.; RUIFENG, I. 2023. Transforming industrial solid waste into eco-friendly zeolite material for efficient heavy metal ion stabilization through host-guest combination, Chemical Engineering Research and Design, vol. 196, pp. 656-670. https://doi.org/10.1016/j.cherd.2023.06.027.