04 – HYDROTHERMAL SYNTHESIS OF NANO-ZEOLITE F FROM KAOLIN TAILINGS
Ano 12 (2025) – Número 3 – Mineral Synthesis Artigos

10.31419/ISSN.2594-942X.v122025i3a4AKCC
Amanda K. C. Cancio1, Bruno A. M. Figueira2*, Cassio F. de Araujo2, Renata de S. Nascimento3, Igor A. R. Barreto3
1Postgraduate Program in Society & Environment, Federal University of Western of Pará, Brazil.
2Postgraduate Program in Science and Materials Engineering, Federal University of Pará, Brazil.
3Postgraduate Program in Geology and Geochemistry, Federal University of Pará, Brazil
*Corresponding author: figueiraufpa@gmail.com
Received on June 29, 2024; after revisions, accepted on December 12, 2024.
ABSTRACT
Kaolin tailings, from Amazon Region (Brazil), were employed as starting material to obtain zeolite F-type material under low-temperature hydrothermal synthesis in alkali solution with KOH. The effects of K/Al ratios, temperature and crystallization time on the crystalline products were investigated. The materials were characterized using X-ray diffractometry, scanning electron microscopy and transmission electron microscopy. The results showed that the kaolin tailings were successfully transformed into nano-zeolite F, with a high degree of crystallinity and morphology in nanorods.
Keywords: Amazon, mining residues, transformation, zeolite.
INTRODUCTION
Kaolin tailings are by-products with no economic value generated during the mineral process that aims to concentrate kaolinite for industrial applications in the manufacture of paper, ceramics, cosmetics, fiberglass, paint and coating, among others (Habashi, 1997; Murray, 2000; Menezes et al., 2014). In the Amazon Region (Brazil), they are abundant and characterized by high contents of SiO2, Al2O3, Fe2O3 and TiO2, as well as the presence of clay minerals such as kaolinite, illite and/or muscovite, hematite and anatase (Paz, Angelica and Neves, 2010; Castro, Maia and Angelica, 2019; Goncalves and Maia, 2018).
These mines and ore deposits still produce by-products with no commercial value through their beneficiation processes. For example, the kaolin industry generates a by-product characterized by its high iron content, and particle size of over 2 mm (Paz, Angelica and Neves, 2010; Castro, Maia and Angelica, 2019; Goncalves and Maia, 2018). These tailings display chemical and mineral properties to be used as a starting material for obtaining various types of value-added products, such as construction (obtaining concrete blocks or replacing sand in sand and cement blocks); production of ceramics, tiles and porcelain tiles, adsorbents and catalysts (Karle et al., 1996; Basaldella et al.,1998; Rezende et al., 2006; Menezes et al., 2007; Maia et al., 2007; Acorsi et al., 2009; Anjos & Neves, 2011; Sousa et al., 2016). In addition, since the mid-2000s they have been proposed as a starting material to produce molecular sieves such as sodalite, faujasite, zeolite A, zeolite P, SAPO-34 and MCM-41 (Paz, Angelica e Neves, 2010; Castro, Maia e Angelica, 2019; Goncalves and Maia, 2018; Pinheiro et al., 2020; Santos et al., 2018).
In this work, we present the transformation of kaolin tailings from Amazon into zeolite F-type material, which is characterized by its structure formed by tetrahedrons of SiO4 and AlO4 with K+ as counterbalance cations based in natrolite tectosilicate mineral (Baerlorcher and Barrer, 1974).
MATERIAL AND METHODS
Raw material
The raw material used in this work comprised kaolin tailings (Ktail) located in the municipality of Vitória do Jari (Amapá state). As a mineralizing agent in the synthesis of zeolite, potassium hydroxide (NEON) was used and deionized water.
Experimental procedures
In the zeolite F synthesis system, initially, kaolin (Ktail sample) was calcined at 700 ºC for 2 h (MTK sample) before it was used. The metakaolin phase (MTK sample) reacted with potassium hydroxide and water at a molar ratio 0.07K2O:0.02Al2O3:0.03SiO2:1H2O with K/Al molar ratio of 3.5. The gel obtained was stirred for 2 h and transferred into hydrothermal reactor at 110º C for 24 hours. The final product was rinsed with distilled water, dried for 2 h at 70º C. The effects of the K/Al molar ratio and temperature on the crystallization of zeolite F were also investigated.
Characterization
The chemical composition of the KTail was determined using a Bruker S2 Ranger X-ray Fluorescence spectrometer, with a Pd tube, using tablets pressed at 20 tons. The raw material and synthetic products were identified by powder X-ray diffractometry, using a D2 PHASER from Bruker (CuKα = 1.5406 Å) 400 W power, with a Bragg-Brentano geometry in continuous mode, scanning speed of 0.25° /min, using a fast LynxEye detector as the detection system. The voltage was 30 kV and 10 mA, respectively. The morphology characterizations were carried out by a TESCAN Vega microscope with EDS analyzer (SEM analysis) and Morgagni 268D equipment (TEM analysis) from Center for Strategic Technologies of the Northeast (CETENE-RECIFE).
RESULTS AND DISCUSSIONS
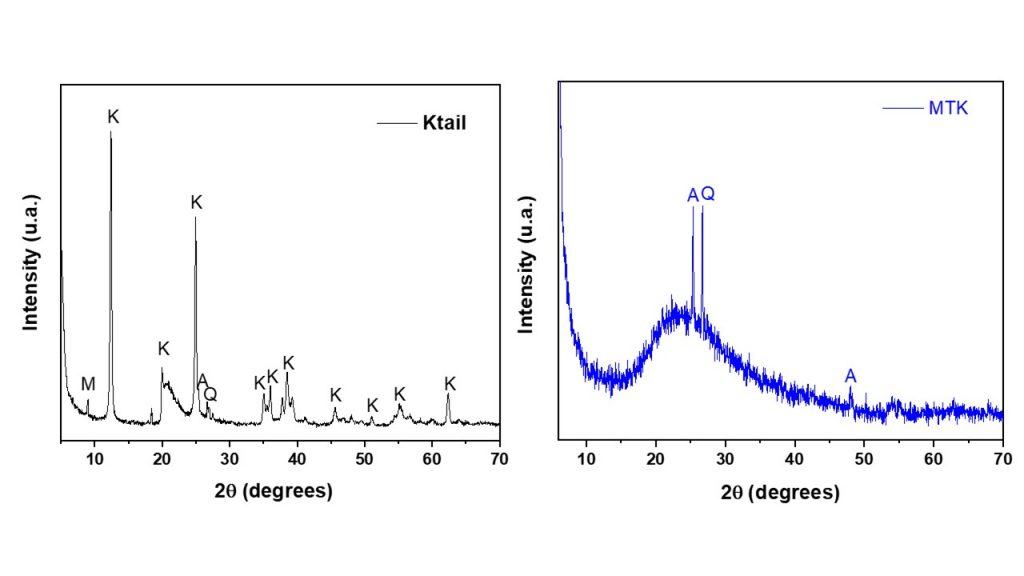
The chemical and mineralogical composition of Ktail employed in this study are shown in table 1 and figure 1. The major constituents were SiO2 and Al2O3, and other oxides, such as Fe2O3, K2O, TiO2 and CaO occurred in lower concentration. The molar ratio of SiO2/Al2O3 was approximately 2.05, which was satisfactory for the synthesis of zeolite F with no additional sources of silica and alumina. The XRD pattern of the starting material (Ktail sample) corresponded to muscovite and kaolinite as aluminosilicate phases, in addition to gibbsite, quartz and anatase which were also detected. The XRD pattern of the MTK sample taken by calcining kaolin at 700ºC, showed that the strong ordered layer structures (kaolinite and muscovite) collapsed to disordered phase (metakaolinite), in accordance with the previous results (Schwanke et al., 2022; Lima et al., 2023). Quartz and anatase were also detected in the sample, remaining as stable phases.
Figure 1- XRD patterns of KTail and MTK samples. (A: anatase; K: kaolinite; M: muscovite; Q:quartz).
Table 1 – Chemical composition of KTail obtained by XRF measurement.Tabela 1 – Chemical composition of KTail
| Composition
(Wt %) |
SiO2 | Al2O3 | Fe2O3 | CaO | K2O | TiO2 | LOI | Total |
| 44.85 | 36.91 | 2.02 | 0.02 | 1.04 | 1.89 | 13.98 | 100 |
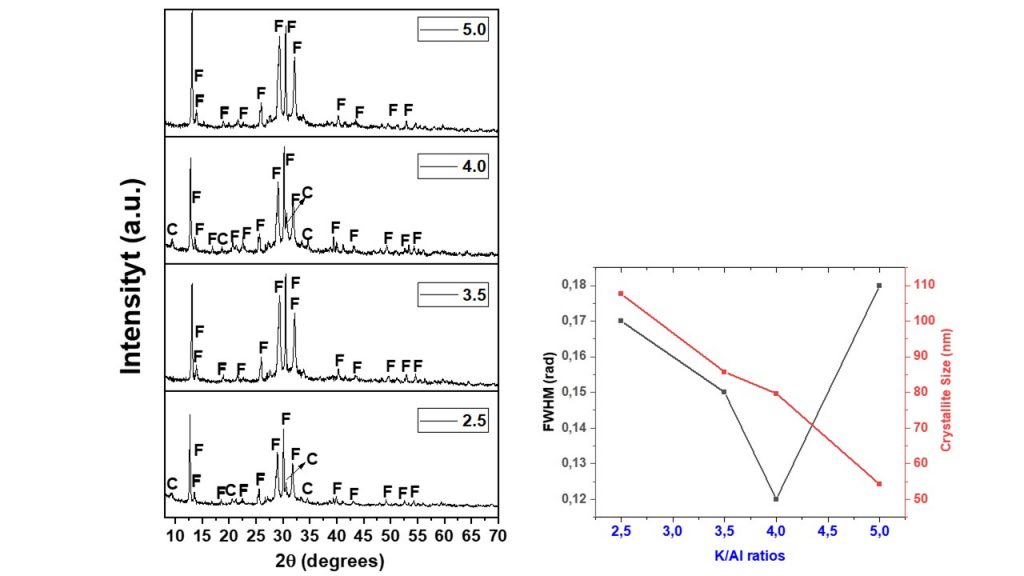
To investigate the role played by varying the K/Al molar ratio in the crystallization of zeolite F, four experiments were carried out with K/Al ratios of 2.5, 3.5, 4 and 5, respectively (Fig. 2). The results indicated an effective transformation of metakaolinite into zeolite F. For the products synthesized under K/Al ratio = 2.5 or 4, in addition to well-defined peaks of zeolite F (PDF 038-0216), K-chabazite-type zeolite (PDF 044-0250) was also verified. On the other hand, an increase in K/Al = 3.5 or 5, produces a pure zeolite F, based on XRD peaks at 12.83; 13.66; 16.88; 18.62; 25.98; 27.58; 29.44; 30.50; 32.17 and 40.27º (2θ), which corresponds to planes (110), (002), (112), (211), (220), (203), (222), (302), (312) and (412), respectively. Except for the sample obtained from K/Al = 5, there was a direct relationship between an increase in structural ordering (measured from FWHM) and a decrease in crystallite size (measured from the Debye-Scherrer equation) for zeolite F phase when there was an increase in the K ratio /Al = 2.5 to 4. For the synthesized product K/Al = 5, a continued reduction in crystallite size (~ 54 nm) was observed against an increase in zeolitic structural disorder.
Figure 2 – XRD patterns of the synthesized zeolites under K/Al ratios at 2.5; 3.5; 4 and 5. And relation between a crystallite size & FWHM. (Zeo F = zeolite F, C = chabazite).
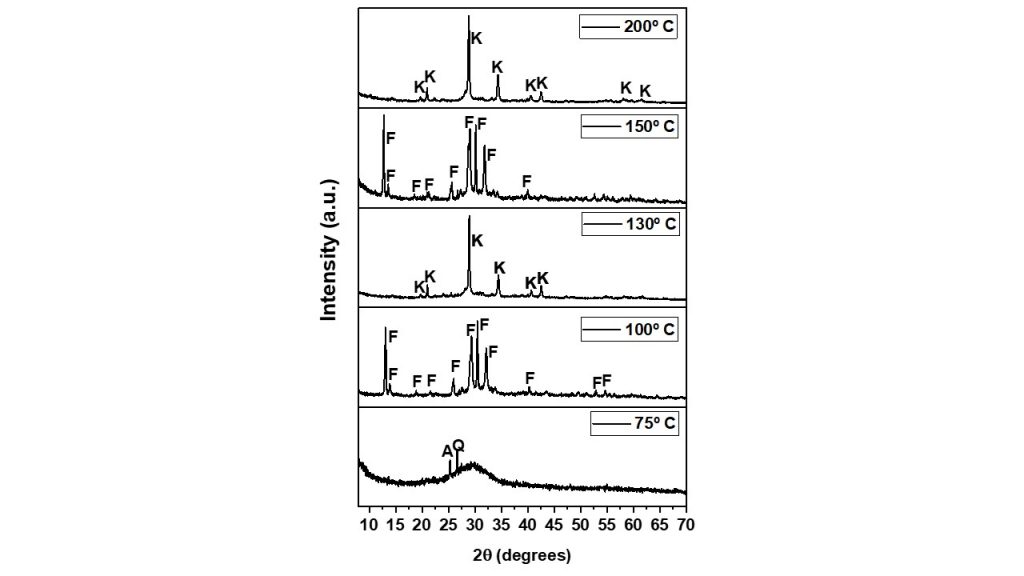
Figure 3 presents the XRD patterns of synthesis products obtained at different temperatures, under hydrothermal treatment after 24 h crystallization. The XRD pattern of the product obtained at 75º C clearly indicated that the synthesis of zeolite F did not occur, as revealed by the presence of amorphous phases, quartz and anatase. If the temperature was around 100 or 150 °C, crystallization results in products with pure phase of zeolite F. When the experiments under the same conditions were carried out at 130 and 200 °C, it was also clear that the amorphous material dissolved and recrystallized into kaliophilite as a crystalline phase. It is interesting to mention that these results agree with those obtained by Novembre, Pace and Gimeno (2014) and Taylor et al. (2020), who used diatomite rocks and waste glass as raw materials for synthesizing zeolite F, respectively.
Figure 3 – X-ray diffraction patterns of the synthesis run at 75, 100, 130, 150 and 200º C. (Zeo F = zeolite F, A = anatase, Q = quartz, K = Kaliophillite).
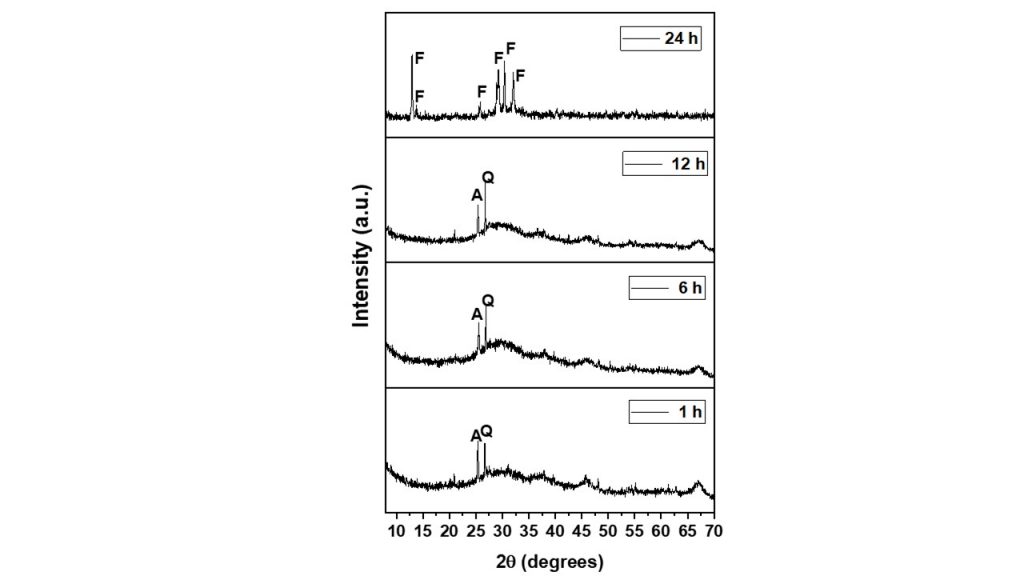
Crystallization time played a crucial role in synthesis of various zeolite-type materials. As shown in figure 4, XRD analyzes of the products synthesized in 1, 6, 12 and 24 hours at 110 °C were used to monitor the minimum ideal time for obtaining zeolite F. For the product obtained at 1, 6 and 12 hours, an almost amorphous material was observed with discrete peaks at 25.39 and 26.71º (2θ) corresponding to the anatase and quartz phases. After 24 hours of crystallization time, the product obtained was characterized as well-crystallized zeolite F, which indicated that it was an adequate time to obtain the potassium zeolitic material and shorter than that observed by Novembre et al. (2014), who reported an ideal crystallization time between 30 and 50 h.
Fig. 4 – X ray diffraction patterns of samples obtained at different synthesis times. (a) 1 h, (b) 6 h, (c) 12 h, (d) 24 h. (Zeo F = zeolite F, A = anatase, Q = quartz).
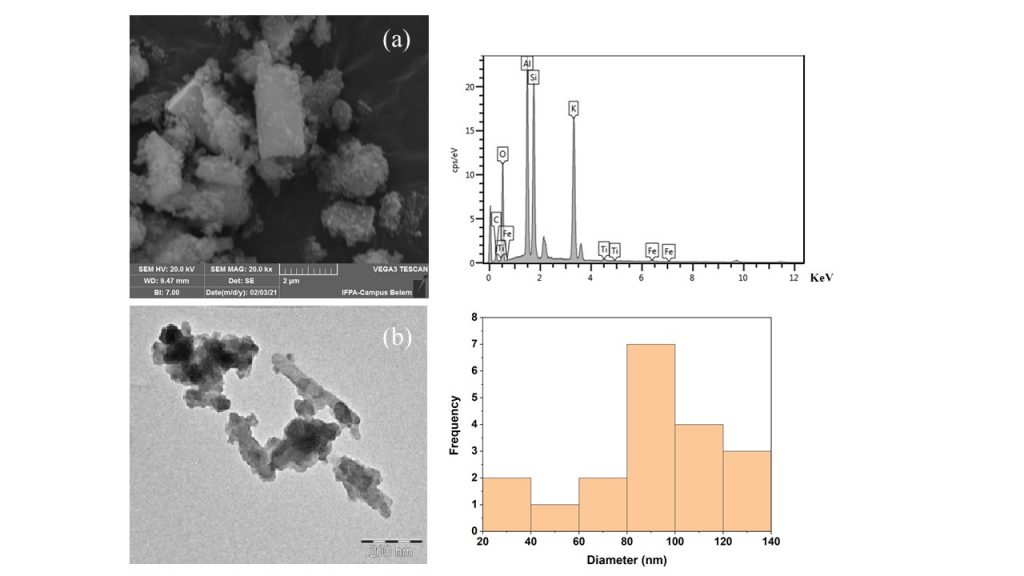
The SEM and TEM micrographs of zeolite F synthesized at K/Al = 3.5 and 110º C for 24 h are displayed in figure 5. The SEM micrograph (Fig. 5a) revealed the presence of irregularly shaped particles in the sample studied with an average size of 2.5 mm. The EDS analysis displayed K, Al, Si, and O atoms and are in accordance with the chemical composition of zeolite F. When the material was characterized morphologically by TEM, the agglomeration of particles in rods was also evident and is 90 nm large (Fig. 5b).
Figure 5 – SEM (a) and TEM (b) micrographs of the synthesized zeolite F.
CONCLUSIONS
Zeolite F-type material can be synthesized from the kaolin tailing from Amazon Region therefore from a starting material, as a principle of low cost. The synthesis parameters as time, crystallization temperature and KOH/metakaolin ratio on the crystalline end products were studied. The results showed that well-developed nano-sticks of zeolite F could be synthesized after 24 h and 110 °C, while the higher temperatures (130 °C) result in kaliophilite -type product.
REFERENCES
ACORSI, M.M.; Schwanke, A.J.; PENHA, F.G.; Pergher, S.B.C.; Petkowicz, D.I. 2009. Transformação de Caulim em Zeólita Tipo P. Cerâmica Industrial, v. 14: 28-33. https://www.ceramicaindustrial.org.br/journal/ci/article/5876573e7f8c9d6e028b4772.
ANJOS, C.M.; NEVES, G.A. 2011. Utilização do resíduo de caulim para a produção de blocos solo- cal. Revista Eletrônica de Materiais e Processos. v. 6 (2): 91-96. https://remap.revistas.ufcg.edu.br/index.php/remap/article/viewFile/228/199.
BAERLOCHER, C. H.; BARKER, R. M. 1974. The Crystal Structure of Synthetic Zeolite F. Zeitung für Kristallographie – Crystalline Materials. v. 140: 10– 26. https://doi.org/10.1524/zkri-1974-1-203.
BASALDELLA, E.I.; KIKOT, A.; TARA, J. C. 1997. Effect of aluminum concentration on crystal size and morphology in the synthesis of NaAl zeolite. Materials Letters. v. 31, pp. 83-86. https://doi.org/10.1016/S0167-577X(96)00256-X.
CASTRO, P.R.S.; MAIA, A.A.B.; ANGELICA, R.S. 2019. Study of the Thermal Stability of Faujasite Zeolite Synthesized from Kaolin Waste from the Amazon. Materials Research. v. 22: 1-7. https://doi.org/10.1590/1980-5373-MR-2019-0321.
GONÇALVES, L.B.; MAIA, A.A.B. 2018. Síntese de sodalita a partir de rejeito de caulim da Amazônia através de extrusão da mistura reacional seguido por processo hidrotermal. BOMGEAM (Boletim do Museu de Geociências da Amazônia), 2: 1-7. DOI: 10.31419/ISSN.2594-942X.v52018i2a10ALG.
HABASHI, F. 1997. Handbook of Extractive Metallurgy: Primary Metals, Secondary Metals, Light Metals. Volume II, ed 1st., Wiley-VCH, Heidelberg.
KARLE, B.G.; BRINKER, C.; PHILLIPS, M. 1996. Zeolite membranes from kaolin. MRS Online Proceedings Library. v. 431: 237-244. https://www.osti.gov/servlets/purl/266618.
LIMA, G.A.; ARAÚJO, C.S.S.; MIRANDA, AM.S., FIGUEIRA, B.A.M. 2023. Síntese hidrotermal por fusão alcalina de zeólita na-p1 de rejeitos de caulim da Amazónia e sua aplicação na retenção de azul de metileno. Holos. v. 6: 1-19. https://www2.ifrn.edu.br/ojs/index.php/HOLOS/article/view/13911/3884.
MAIA, A.A.B.; SALDANHA, E.; ANGÉLICA, R.S.; SOUZA, C.A.G.; NEVES, R.F. 2007. The use of kaolin wastes from the Amazon region on the synthesis of zeolite A. Cerâmica. v. 5: 319-324. https://doi.org/10.1590/S0366-69132007000300017.
MENEZES, R.R.; ALMEIDA, R.R.; SANTANA, L.; FERREIRA, H.S. 2007. Utilização do resíduo do beneficiamento do caulim na produção de blocos e telhas cerâmicos. Revista Matéria, v. 12 (1): 226 – 236. https://doi.org/10.1590/S1517-70762007000100028.
MENEZES, R. A.; PAZ, S.P.A; ANGELICA, R.S.; NEVES, R.F. 2014. Color and Shade Parameters of Ultramarine Zeolitic Pigments Synthesized from Kaolin Waste. Materials Research. v. 17: 23-27. https://doi.org/10.1590/S1516-14392014005000078
MURRAY, H. H. 2000. Traditional and new applications for kaolin, smectite, and palygorskite: a general overview. Applied Clay Science. v. 17: 207-221. https://doi.org/10.1016/S0169-1317(00)00016-8.
NOVEMBRE, D.; PACE, C.; GIMENO, D. 2014. Syntheses and characterization of zeolites K-F and W type using a diatomite precursor. Mineralogical Magazine. v. 78:1209–1225. https://doi.org/10.1180/minmag.2014.078.5.08.
PAZ, S.P.A.; ANGELICA, R.S.; NEVES, R.F. 2010. Síntese hidrotermal de sodalita básica a partir de um rejeito de caulim termicamente ativado. Química Nova. v. 33: 579-583. https://doi.org/10.1590/S0100-40422010000300017.
PINHEIRO D.R. GONÇALVES, L.R.; SENA, R.L.P.; MARTELLI, M.C.; NEVES, R.F.; RIBEIRO N.F.P. 2020. Industrial Kaolin Waste as Raw Material in the Synthesis of the SAPO-34 Molecular Sieve. Materials Research. https://doi.org/10.1590/1980-5373-MR-2020-0043.
REZENDE, M. L. S.; Araújo, I. F.; Neves, G. A.; Nascimento, J. W. B.; Silva, W. R. 2006. Gerência de resíduos de caulim: estudo da viabilidade para produção e blocos de concreto. In: Simpósio de Engenharia de Produção, Bauru, SP. Anais. Bauru, 2006. https://simpep.feb.unesp.br/anais/anais_13/artigos/179.pdf.
SANTOS, E.C.; Costa, L. S.; Oliveira, E. S.; Bessa, R. A.; Freitas, A. D. L.; Oliveira, C. P.; Nascimento, R. F.; Loiola, A. R. 2018. Al-MCM-41 Synthesized from Kaolin via Hydrothermal Route: Structural Characterization and Use as an Efficient Adsorbent of Methylene Blue. J. Braz. Chem. Soc. v. 29, pp. 2378-2386. https://doi.org/10.21577/0103-5053.20180115.
SCHWANKE, A. J.; SILVEIRA, D. R.; PUTON, B. M. S.; CANSIAN, R. S.; BERNARDO-GUSMÃO, K. 2022. Sustainable conversion of Brazilian Amazon kaolin mining waste to zinc-based Linde Type A zeolites with antibacterial activity. Journal of Cleaner Production. v. 33: 130659. https://doi.org/10.1016/j.jclepro.2022.130659.
SOUSA, J.B.M.; LIMA, A.G.B.; NASCIMENTO, P.H.M.; COSTA, S.S.S. 2016. Aproveitamento de Resíduos de Caulim e Granito na Formulação de Massas Cerâmicas para Fabricação de Grés Porcelanato. In: Congresso Técnico Científico da Engenharia e da Agronomia – CONTECC. Foz do Iguaçu – PR. 2016. http://dspace.sti.ufcg.edu.br:8080/jspui/handle/riufcg/2065.
TAYLOR, J. H.; ELMES, V. K.; HURT, A. P.; COLEMAN, N. J. 2020. Synthesis of feldspathoids and zeolite K–F from waste amber container glass. Materials Chemistry and Physics. v. 246: 122805. https://doi.org/10.1016/j.matchemphys.2020.122805.