07 – PHOSPHORUS FRACTIONS IN POTTERY SHERDS FROM AMAZONIAN DARK EARTH
Ano 06 (2019) – Número 02 Artigos
![]() 10.31419/ISSN.2594-942X.v62019i2a7GISSV
10.31419/ISSN.2594-942X.v62019i2a7GISSV
Glayce Jholy Souza da Silva Valente1, Marcondes Lima da Costa2
1PNPD-PPGG, Instituto de Geociências da Universidade Federal do Pará, glaycej@yahoo.com.br;
2Instituto de Geociências da Universidade Federal do Pará, Pesquisador do CNPq e Membro Titular da ABC, marcondeslc@gmail.com
ABSTRACT
The aim of this study was to investigate phosphorus (P) fractions in pottery sherds from Amazonian Dark Earth. Pottery sherds samples were collected from three archaeological sites: Raimundo (Caxiuanã-Pará), Quebrada Tacana (Letícia-Colômbia) and Juruti (Baixo Amazonas – Pará). The results showed that Juruti sherds had a highest contribution to the total inorganic phosphorus. Analysis of different inorganic phosphorus fractions indicates the greatest presence of phosphates bonded to aluminum all the sherds.This difference between the pottery sherds may be attributed to their mineralogical and non-plastic composition.
Keywords: inorganic P, organic P, sequential extraction, mineralization
INTRODUCTION
Archaeoantrosols known as Archaelogical Dark Earth (ADE) occur as dark and irregular patches widespread in the Amazon Basin, overlapping different geomorphological surfaces positioned in the landscape to the nearest water sources. Associated with these soils, pottery sherds are distributed on their pretic horizon related to pre-Columbian human activities, as well as lithic instruments and visible remnants of charcoal, with less repercussion. Over the last few years, investigations focused on pottery sherds have revealed them as a collaborative tool in the study of ADE.
Given the well-established particular features for these soil types, the pottery sherds also show that rescued in different locations the use of a common raw material represented in its essence by kaolinite (metakaolin) and quartz in which the other variations are due of non-plastics used according to local availability or even need subdued by those who made it as a functional piece.
Like soils, pottery sherds still retain a fertile character, especially regarding their phosphorus amounts, regardless of their chemical representativeness, whether total or availability phosphorus content (accessible to plants) (Valente and Costa 2017). It has long been discussed about the origin of phosphorus in pottery sherds, in which some authors such as Freestone et al. (1994) pointed this origin as a post-depositional due to enviromental alterations during burial, however, Rodrigues et al. (2015) and Rodrigues & Costa (2016) demonstrated phosphorus as evidence of use, whose absorption occurred during protein food processing stimulated by heating in a fully reactive medium.
In order to understand studies involving the phosphorus content known to be contained in pottery sherds, this work aims to quantify through conventional extraction and fractionation methods the contents of the main P: organic (Po) and inorganic (Pi) fractions including their complexes. Al, Fe, Ca and P-H2O (or poorly adsorbed) for Pi.
MATERIALS AND METHODS
Pottery sherds samples were collected from three archaeological sites distributed over of Amazon. Juruti site (2°10’01.68”S/56°05’57.58”O, TP1) e (2°10’36.86”S/56°06’17.05”O, TP2), Quebrada Tacana site (4°07’09.1”S e 69°55’16.1”O) and Raimundo site (01°45’36.00”S e 51º26’34.3”O). The fractionation procedures are based on the differential solubilities to establish different form of phosphorus extracted by using selective chemical extractants. As there are no phosphorus fractionation exclusive methods to the pottery sherds, classical procedures of speciation applied to soils were used in this approach.
The sequential extraction was carried out for phosphorus fractions with powdered pottery sherds samples. The organic phosphorus was determined according to Bowman and Moir (1993) method. Fractionation of inorganic phosphorus was based on the method proposed by Chang and Jackson (1957) and subsequently modified by Kuo (1996). In the first step, soluble or weakly adsorbed phosphorus was extracted (NH4Cl 1 mol L-1), in the second step, Al- and Fe-bound phosphates are separated, NH4F/NaCl e NaOH/NaCl/H2SO4, respectively. The fraction of phosphates bonded to calcium is extracted in third step (H2SO4 extracted), whereby detection was performed spectrophotometrically at the wavelength of 830 nm in a UV/VIS spectrophotometer using the phosphomolybdate-blue-method (Murphy and Riley 1962).
RESULTS AND DISCUSSION
When associating the phosphorus content in pottery sherds with the use of vessels in the past, like food preparation and consumption, it is understood that its origin comes from both compounds containing -CH bonds, coming from blood and fat, in which fat may have a contribution of vegetable origin, as well as compounds with bonds P-Ca, attributed to bones, spines and carapaces essentially of animal origin.
Based on the nature of the phosphorus bond, organic phosphorus compounds is classified into phosphate esters, phosphonates and phosphoric acid anhydrides. Phosphate esters are subclassified into monoesters and diesters according to the number of ester linkage to orthophosphate. Phosphate monoesters occur mainly as inositol phosphates and phosphate diesters include nucleic acids (DNA and RNA), phospholipids and teichoic acids (Turner et al. 2005, Nash et al. 2014).
In soils a large portion of organic phosphorus is stabilized by association with mineral components. Organic phosphorus compounds (negatively charged) bind directly or through polyvalent cations, such as calcium or ferric iron, to soil clay fraction minerals and hydrous iron or aluminum oxides (Turner et al. 2005, Zhu et al. 2017). Featuring a highly reactive matrix, represented by metakaolin (metastable phase of kaolinite), and sometimes cations from minerals present in materials used as non-plastics, sometimes the mineral itself, to examples Ca constituents of aragonite / calcite (CaCO3 ) of shells, goethite / hematite / magnetite Fe, among others represented by ETRs.
Experimentally, crystalline phases of the variscite (AlPO4.2H2O) were formed on the walls and lids of non-archaeological ceramic vessels under simulated cooking simulations (Rodrigues & Costa 2016). The results expressed in the present work comprised analysed data from archaeological material, and possibly portray the phosphorus forms adsorbed in the containers until the removal of their primary function, since Rodrigues (2010) and Valente & Costa (2017) demonstrated how the process of P desorption in pottery sherds is favorable and occurs slowly, especially non-crystalline phosphates, such as is the case of pottery sherds investigated in the present work.
The highest value of phosphorus fraction was found as organic in the pottery sherds of the investigated sites, except for the set belonging to the Juruti site (Figure 1), where the contribution of the inorganic fraction was higher, reaching almost 70% of the total P. These distinctions show the different stages in the P mineralization process for the sherds. In which much of the organic P content initially introduced into the walls and lids of ceramic vessels through cooking of animal or vegetable origin has been solubilized by converting to inorganic phosphate, becoming labile to plants (Nash et al. 2014, Darch et al., 2016).
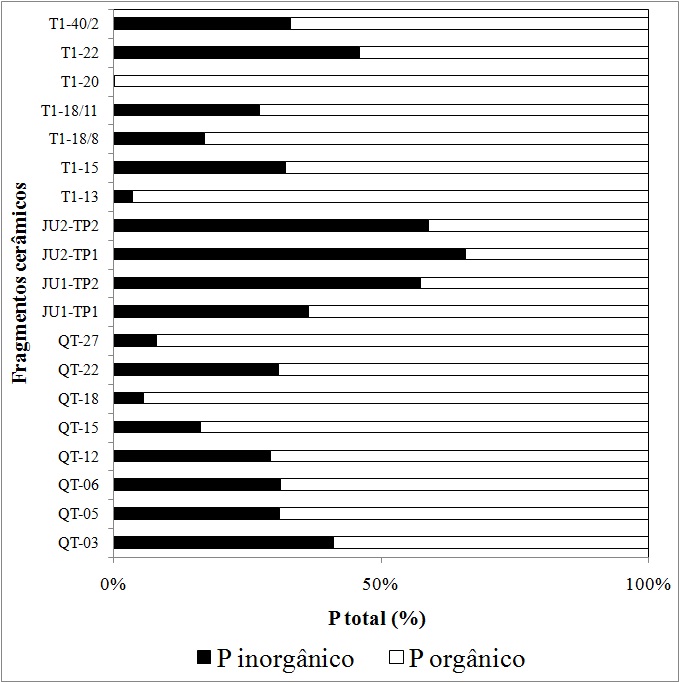
Figure 1. Percentage distribution of inorganic total phosphorus (Pi) and organic (Po) relative to the total phosphorus content (Pt). Sherds from Raimundo site: T1-40/2, T1-22, T1-20, T1-18/11, T1-18/8, T1-15, T1-13. Sherds from Juruti site: JU2-TP2, JU2-TP1, JU1-TP2, JU1-TP1. Sherds from Quebrada Tacana site: QT-27. QT-22, QT-18, QT-15, QT-12, QT-06, QT-05, QT-03.
Extraction and determination of different fractions of inorganic phosphorus indicate the sequence in the recovery rate of P. Analysis of different inorganic phosphorus fractions indicates predominance of phosphates bonded to aluminum – NH4F/NaCl for all sherds. For the sherds from Raimundo site, the greatest presence of phosphates bonded to calcium-H2SO4 was obtained (Figure 2).
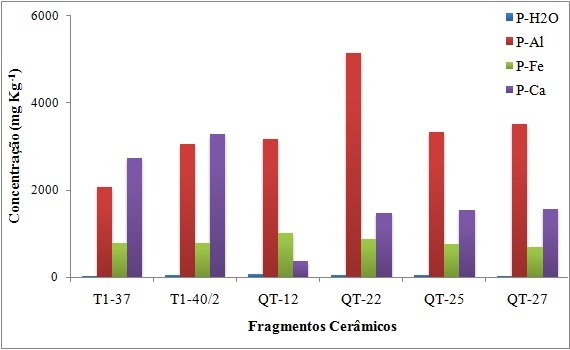
Figura 2. Distribution of Pi fractions in the sherds from Raimundo site (T1-37 e T1-40/2) and Quebrada Tacana (QT-12, QT-22, QT-25 e QT-27).
Reactions involving phosphate ions with iron hydroxides occur under conditions adverse to those occurring in pottery sherds (Toledo 1999, Milić et al. 2019). This justifies the lower representativeness for these compounds which is followed by weakly adsorbed phosphorus or P-H2O. And just as the availability of Al promotes the formation of aluminum phosphates, phosphate formation with CaCO3 occurs for the Raimundo site sherds, whose source of Ca comes from shells used as non-plastics in the clayey matrix, evidenced by the naked eye, by optical microscopy and mineralogically portrayed as calcite / aragonite (Valente et al. 2013, Valente et al. 2017).
The equivalence in the recovery rate of Al and Ca-bonded phosphates for the Raimundo site sherds indicates a possible formation of crandallite phosphates, as evidenced by Rodrigues et al. (2015), in pottery sherds from the Bragantina region (PA), which presented the same sources of Ca.
CONCLUSIONS
The results yielded the chemical behavior of phosphorus in pottery sherds resembles those commonly represented in lateritic materials, tropical soils and sediments. From an organic source of P, the orthophosphate ion tends to seek chemical stability by complexing with available cations from the surrounding environment, where the most common and abundant are Al, Fe and Ca. It also showed differentiated mineralization potential for the sherds of distinct sites. Although no mineral representative, the Juruti site sherds had significantly higher proportion inorganic phosphorus compared to the organic fraction, dominant in other sites sherds.
Acknowledgements
We are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) or the fellowships granted and supporting the project “Geochemical Signatures for the Identification of Amazonian Black Earth” (process number 484986-2007-4; Grant Nr. 305015/2016-8). We also thank Scientia Consultoria Ltda. and Prof. G. M. Rios (Universidade Nacional da Colômbia) for the donation of samples analyzed in the present study and Chemistry Anlaysis Laboratory (IG-UFPA) for the technical support.
REFERENCES
Bowman, R. A. and Moir, J. O. 1993. Basic EDTA as an extractant for soil organic phosphorus. Soil Sci. Soc. Am. J., 57: 1516-1518.
Chang, S.C. and Jackson, M.L. 1957. Fractionation of soil phosphorus. Soil Sci., 84:133-144.
Darch, T., Blackwell, M. S.A., Chadwick, D., Haygarth, P. M., Hawkins, J.M.B., Turner, B. L. 2016. Assessment of bioavailable organic phosphorus in tropical forest soils by organic acid extraction and phosphatase hydrolysis. Geoderma, 284: 93–102.
Freestone, I.; Middleton, A.; Meeks, N. 1994. Significance of phosphate in ceramic bodies: discussion of paper by Bollong et al. Journal of Archaeological Science, 21: 425-426.
Kuo, S. Phosphorus. In: BIGHAM, J.M. Methods of soil analysis: chemical methods. Madison, Soil Science Society America/American Society of Agronomy, 1996. Part. 3. p.869-919.
Milić, S., Ninkov, J., Zeremski, T., Latković, D., Šeremešić, S., Radovanović,V., Žarković, B. 2019. Soil fertility and phosphorus fractions in a calcareous chernozem after a long-term field experiment. Geoderma339: 9–19.
Murphy, J. and J.P Riley. 1962. A modified single solution method for the determinationof phosphate in natural waters. Anal. Chim. Acta. 27: 31-36.
Nash,D. M., Haygarth,P. M. Turner,B. L., Condron, L. M., McDowell,R.W.,Richardson,A. E., Watkins, M., Heaven.M.W. 2014. Review. Using organic phosphorus to sustain pasture productivity: a perspective. Geoderma221–222: 11–19
Rodrigues S.F.S., Costa M.L., Pöllmann H., Kern D.C., Silveira M. I. da, Kipnis R. 2015. Pre-historic production of ceramics in the Amazon: Provenience, raw materials, and firing temperatures. Appl. Clay Sci. 107: 145-155.
Rodrigues, S.F.S., Costa, M.L. 2016. Phosphorus in archeological ceramics as evidence of the use of pots for cooking food. Appl. Clay Sci. 123: 224-231.
Toledo, M. C.M. 1999. Os Fosfatos aluminosos da serie crandallita- Uma Revisão. Revistado Instituto Geológico IG, 20 (1/2): 49-63.
Turner, B. L., Cade-Menun, B. J., Condron, L. M.; Newman, S. 2005. Extraction of soil organic phosphorus. Talanta, 66: 294–306.
Valente. G.J.S.S., Costa M.L. 2017. Fertility and desorption capacity of Anthrosols (Archaeological Dark Earth-ADE) in the Amazon: The role of the ceramic fragments (sherds). Applied Clay Science, 138: 131-138.
Valente. G.J.S.S., Costa M.L., Rodrigues, S.F.S. 2013. Mineralogia e análise micromorfológica de fragmentos cerâmicos de sítios TPA. Anais do 13º Simpósio de Geologia da Amazônia.
Zhu, J., Qu, B., Li, M. 2017. Phosphorus mobilization in the Yeyahu Wetland: Phosphataseenzyme activities and organic phosphorus fractions in the rhizospheresoils. International Biodeterioration & Biodegradation, 124:304-313.
