03 – SEDIMENTARY MANGANESE DEPOSITS IN CARAJÁS, BRAZIL
Ano 09 (2022) – Número 02 Artigos
![]() 10.31419/ISSN.2594-942X.v92022i2a3MLC
10.31419/ISSN.2594-942X.v92022i2a3MLC
Marcondes Lima da Costa1*
Oscar Jesus Choque Fernandez2
Carlos Eduardo Reinaldo Delgado3
Luiz Cláudio Gonçalves da Costa4
Marlis Elena Ramirez Requelme2
1Federal University of Pará, Belém, PA, Brazil, marcondeslc@gmail.com
2Federal Institute of Technolgy Pará, Belém, PA, Brazil, ochoque.fernandez@gmail.com, marliselena@yahoo.com.br
3Vale S.A, Carajás, Brazil, carlos.delgado@vale.com
4Vale S.A. at the time the research was carried out
*Corresponding Author
Highlights:
- Lenses of carbonaceous and Mn-oxides-rich shales hosted by reddish siltstones;
- Fine amorphous Mn-oxides deposited in a shallow platform approximately 2.0 Ga;
- Stromatolite-like structures rich in Mn-oxyhydroxides;
- Tectonic-hosted Mn-oxyhydroxides + kaolin + pyrite; and
- Cryptomelane and cryptomelane-hollandite are the main Mn ore minerals.
GRAPHIC ABSTRACT
ABSTRACT
The Carajás Mineral Province in Brazil is one of the most important in the world because it contains world-class mineral deposits (Fe, Cu, Au, Ni and Mn), with Fe, Ni and Mn being mainly of lateritic enrichment. In Carajás, at least four distinct Mn deposits are known (Azul, Buritirama, Buriti and Sereno), and the best known and still important are represented by the Igarapé Azul. The main mines are located in Azul and Buritirama. The Azul deposits are the earliest known and were initially considered as typically lateritic. Further studies have shown that much of the ore manganese reserves are classically associated with carbonaceous grey to black shales represented by oxyhydroxides, where cryptomelane and cryptomelane-hollandite are the major ore mineral. All together as large lenses, they are hosted by thick packets of reddish siltstones and sandstones of Paleoproterozoic (approximately 2.1 Ga). The entire sedimentary package was not affected by metamorphism, perhaps at most anchimetamorphism, as suggested by the discreet presence of sericite and partly chlorite, which can also be diagenetic. The mineralogy dominated by Mn-oxyhydroxides and whole chemical composition of rocks and ore and specific geochemical diagrams after them, as well as the abundance of carbonaceous organic matter and the presence of stromatolite-like structures, show deposition in a shallow marine platform. The source of manganese is both hydrogenous and hydrothermal. The incursion of hydrothermal Mn contribution, in part abyssal, is indicated by high concentration of Co-Ni-Cu and Ba, as well as REE and slight positive Ce and Eu anomalies. Rhodochrosite is restricted and early diagenetic, likely pyrite, as confirmed by the distinct isotopic data. The deposition of Mn oxides, mainly amorphous, appears to be microbial mediated, probably silicium bacteria (Si-O-Mn-C), and close to ocean redoxcline, being mostly oxic conditions. Most of the crystalline Mn-oxyhydroxides are of diagenetic origin and in part epigenetic. The restricted presence of carbonates and the marked presence of carbonaceous organic matter shows that, during the diagenesis, the original conditions of sedimentation prevailed, only locally reducing, when rhodochrosites and pyrites were formed. This is reinforced by the isotopic composition of the organic matter. Tectonic deformations reached the entire package during two regional events (Trans-Amazonic and Brazilian) and provided the remobilization and reprecipitation of Mn-oxyhydroxides of high content and crystallinity (pyrolusite and manganite) along faults, fractures and apical folds.
All these data show that the Mn deposits of Azul find parallels in several world deposits formed in the first Great Oxidation Event of the Earth’s atmosphere, between 2.4 and 2.1 Ga. They also find some parallels in the deposits of Neoproterozoic until Cenozoic. The great differential is the mastery of Mn oxyhydroxides and the absence of metamorphism, when confronted with the older ones.
Keywords: cryptomelane; hollandite; stromatolite-like structures; carbonaceous organic matter; stable isotopes; tectonic-hosted Mn ores.
INTRODUCTION
The strong economic and industrial development of Asian countries, especially China and India, has increased the demand for manganese ores sharply, without considering that some famous mines, such as Serra do Navio and Conselheiro Lafaiete in Brazil have run out. Brazil, until then one of the main players of Mn, was restricted in general terms only to the mines of the Azul and Buritirama (Salgado et al., 2019) in Carajás Mineral Province (CMP) (Figure 1), and Urucum in Mato Grosso do Sul (Biondi & Lopez, 2017), besides small deposits in Bahia. In view of this global and national framework, it was imperative to develop geological studies to understand the genesis of manganese deposits, aiming to increase the reserves of the known deposits and discover new ones.
The manganese ore of the Azul (Figure 1) was considered to be of lateritic origin from alteration of rhodochrosite-rich proto-ore related to two rhodochrosite units (Lower and Upper Units) (Figure 2) (Anderson et al., 1974; Valarelli et al., 1978; Bernardelli & Beisiegel, 1978, Bernardelli, 1982, Beauvais, 1984, Coelho & Rodrigues, 1986, Beauvais et al., 1987, Silva, 1988, Vasconcelos et al., 1994, Ruffet et al., 1996, Weber, 1997; Dardenne & Schobbenhaus 2001).
Costa et al. (2005), however, showed that the main ore then mined fifteen years ago corresponded to several packets of Mn-oxyhydroxides enveloped by dark grey to black shale lenses. These lenses then converge externally to grey shales hosted by thick packets of red siltstones to sandstones that dominated the entire region of the mine and even its surroundings. They also showed that these rocks were affected by tectonic deformations that favour the formation of Mn-oxyhydroxide mineralizations hosted by faults, folds and veins and strongly associated with kaolin venules. In the end, they proposed that these sedimentary rocks in fact belonged to the formation Águas Claras, with an age approximately 2.1 Ga (Costa et al., 2005; Fabre et al., 2011). They also verified that rhodochrosite was a very restricted mineral. Araújo & Sousa (2018) in a large report, presented a detailed study of the manganese ore deposits in Carajás and its turnaround. For Azul they followed the origin proposed by Costa et al (2005) by using a detailed geological description for the mine front and drill holes, and presented optical mineralogical and texture analyses, XRD and MEV/EDS mineralogical data, and several instances of U-Pb dating using detrital zircons, structural analyses and even geophysical surveys. They then reinforced the sedimentary and supergene importance for manganese ore and admitted that the sedimentary ore is associated with the Archean sequence formed by pelitic rocks. Although manganese deposits involve up to four different formation environments (sedimentary, volcanic-sedimentary, metamorphosed, hydrothermally modified, hydrothermal and supergene), sedimentary deposits are economically the most important and are mainly distributed from the Proterozoic through the Quaternary (Laznicka, 1992; Fan & Yang, 1999). For manganese deposits in the CMP, considered as supergene (lateritic), new research shows that at least the Azul deposits mined in the last 16 years are mainly sedimentary. The estimated ore reserves are of the order of 150 Mt and therefore a deposit of medium size.
In general, the oxide manganese deposits are considered to be formed by supergenic alteration over manganese carbonate or silicates (Weber, 1997; Maynard, 2004), but some deposits, even of large amounts to oxyhydroxides are typically sedimentary (Haralyi & Walde, 1986), such as those of Ukraine, Australia, South Africa (Kuleshov, 2017), and even in part of Gabon (Maynard, 2004).
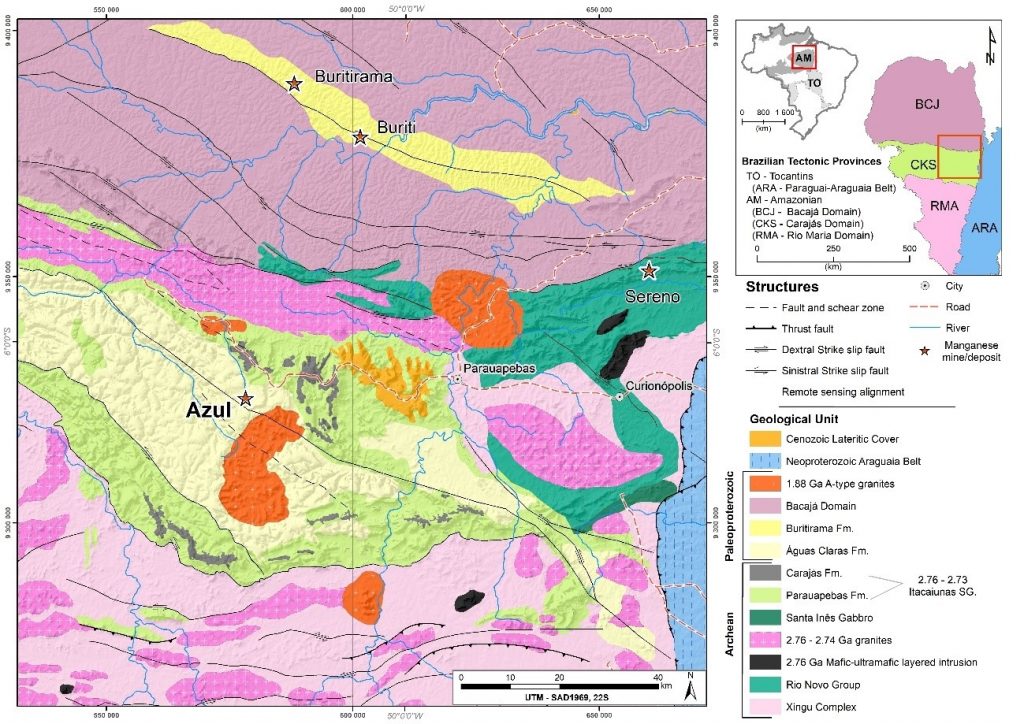
Figure 1 – Geological map of Carajás Mineral Province (CMP) with indication of its main manganese deposits (Azul, Buritirama, Buriti and Sereno). Modified from Vasquez et al (2008).
The manganese of Azul is not, however, a unique manganese deposit in the region of CMP. The next most important is Buritirama located to the north of the Carajás block (Figure 1), bordered by the Bacajá block (Salgado et al., 2019), whose production level is already comparable to that of Azul. Although very rarely investigated from the point of view of its origin the manganese ore is considered to be lateritic (supergene ore) and locally hydrothermal (Araújo & Sousa, 2018; Salgado et al., 2019), formed from carbonate rocks (kutnohorite, rhodochrosite and Mn-calcite) and manganese silicates (rhodonite, spessartine, Mn-diopside) rocks with orthoquartzites and mica schists (Valarelli et al., 1978; Salgado et al., 2019).
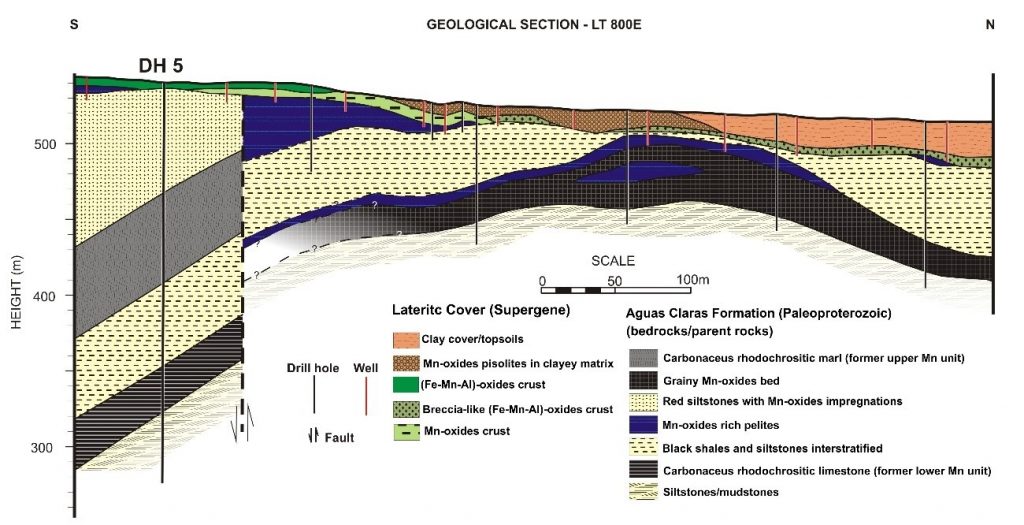
Figure 2 – The first published geological cross section for Azul manganese deposit with indication of the deep drill hole 5 (DH 5) and others after Bernardelli & Beiseigel (1978) and republished by Beauvais et al. (1987).
Buriti is another manganese deposit in CMP, though smaller than the other ones (Figure 1), just south of Buritirama. Here, the ore occupies higher relief and is layered and hosted by compact but friable sandstones. The ore is massive, stratified, dense, and composed mainly by cryptomelane (Figure 3 A and B). The top is covered by colluvium (Figure 3 A) of this ore material. This deposit was not the subject of academic studies.
Finally, there are Mn-oxyhydroxide deposits of Sereno (Figure 1) which occupy the cliffs of a large valley (Figure 3 C) in the northern region of Serra Leste, next to the Serra Pelada domain area, which is distinguished by the richness of gold associated with manganese and carbonaceous shales. Manganese ore locally is very thick and presents as metric pockets massive to cavernous (Figure 3 D), embedded in fine quartzites that are strongly tectonically deformed. The only geological information available on this deposit is reported by Araújo & Sousa (2018), who classified the manganese ore as lateritic.

Figure 3 – Geological expositions at Buriti manganese deposit: (A) Mn-rich colluvium and (B) the massive manganese ore composed by cryptomelane; (C) the wide valley with steep cliffs in the Sreno manganesedeposit; (D) cliff exposition of Mn-oxyhydroxides ore in the Sereno deposit.
The main objective of this work is then to present the dominant presence of the eminently sedimentary and restrictly superimposed tectonic ore mineralizations, on which the lateritic and its degradation (detrital and pisolithic) have been developed, which largely support the current landscape of the Azul Mn deposits in the CMP. It will present new mineralogical data and mineral chemistry, especially for cryptomelane and cryptomelane-hollandite, rock geochemistry and geochronology, isotopic geochemistry and the occurrence of stromatolite-like structures. The new data were only possible after the studies carried out over the last 15 years, through fieldwork on several occasions, including the description of several drilling holes and collection of representative samples.
With this perspective of a sedimentary origin for the manganese ore from Azul, a new environment is opened to prospect new deposits and increase reserves of this ore in the region, where laterite Mn-bearing crusts may be strong geological indicators.
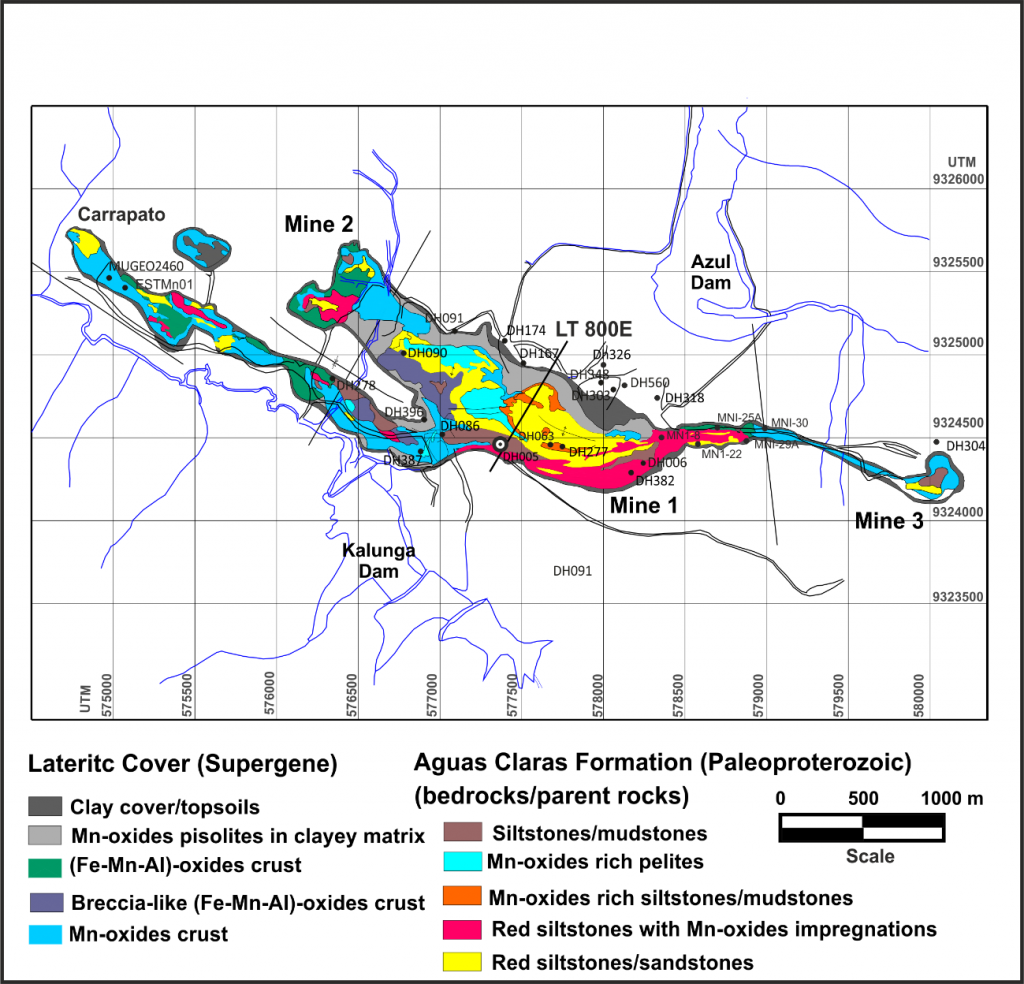
Azul’s manganese deposits are located in the CMP (Figure 1), southeast of the State of Pará. This province comprises relatively elevated terrain for the Amazonian pattern, represented by kilometer-long plateaus, with altitudes varying between 600 and 900 m and valleys and undulating surfaces between the plateaus, watershed, from 300 to 400 m in altitude. The tops of the plateaus are slightly undulated to almost flat. The Azul manganese deposits are located on the slopes of an east-west kilometre plateau with up to 600 m in altitude and exposed in the depth during mining in the last 16 years. The manganese ore also cropped out on the slopes and at the bottom of the valleys of the Azul creek.
An extensive Paleoproterozoic metasedimentary sequence, mainly clastic, marine to fluvial, denominated as the Águas Claras Formation (Nogueira et al., 1995), covers a large Archean to Paleoproterozoic area of CMP (Figure 1). This sedimentary cover carries the manganese ore of the Azul and is, at the same time, the source material of the lateritic manganese ore.
The Águas Claras Formation occupies an area of 900 km2 in the central part of the Carajás Transcurrent System, the primary ore bearer, consisting essentially of shales (siltstones and mudstones) with different degrees of lithification, being locally deformed by faults and folds related to this system in the mineralized zone. The lower mudstone and siltstone members were deposited on a marine platform system, and the sandstones and conglomerates of the upper member are of fluvial and shallow coastal origin (Nogueira et al., 1995).
Most of the surficial terrain of the Azul manganese mines are laid on lateritic materials, mainly arising as iron-aluminum crusts, manganese crusts, or clay-like argisols with Fe and Mn-oxyhydroxide spherulites and fragments (Figures 4 through 7). These laterite formations correspond to old, thick lateritic profiles, equivalent to those that formed part of the iron, aluminium, gold, and nickel ores, among others, in Carajás and Amazonia in general (Costa, 1997; Costa et al., 2005).
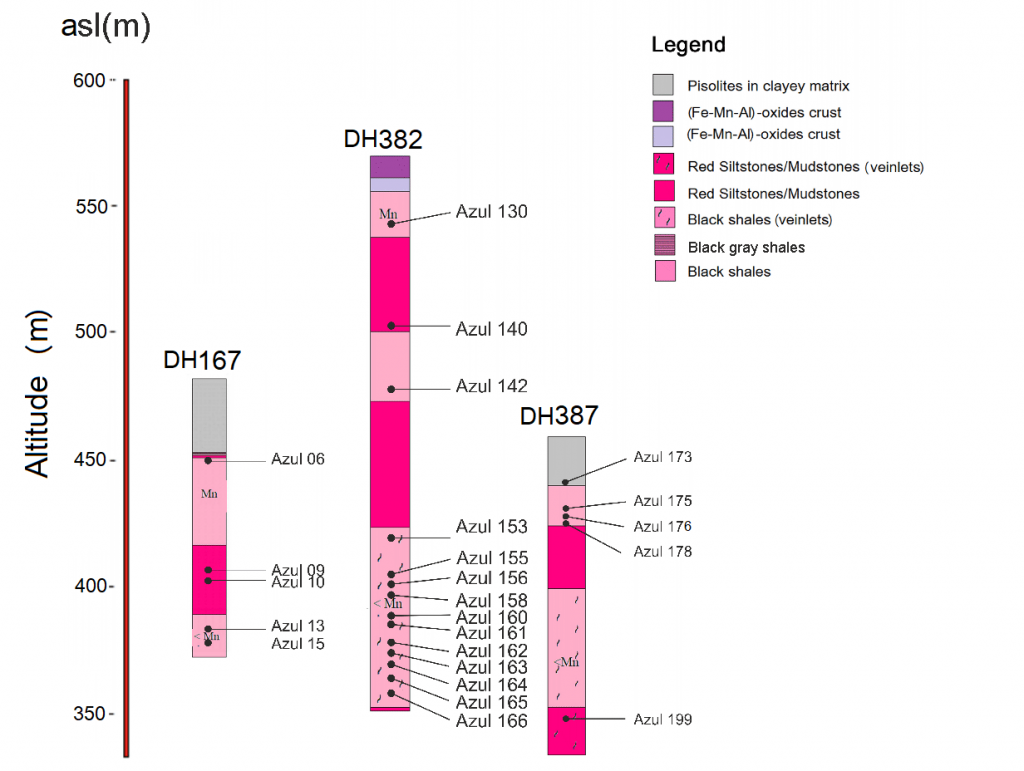
Figure 4 – Geological sections of the drill holes DH 167, DH 382 and DH 387 with indication of the collected samples.
MATERIALS AND METHODS
Fieldwork and sampling – The fieldwork consisted of several mapping trips in and out of the pits, describing shallow to 200- m in depth boreholes (Figure 4). Sampling was carried out on the mining fronts and along the drill holes (Figures 4 and 5, Table 1), representing the materials of the lateritic profiles and sedimentary rocks and their products of tectonic deformation, mainly along faults, folds, and fractures.
Optical Microscopy – It was used for mineralogical identification and textural analysis on both thin and polished sections for the different minerals, in which opaque (manganese ore minerals) are dominant. For the identification of the mineralogical phases, a ZEISS microscope, model AXIOLAB POL, with reflected light, ocular of 2.5x, 10x, 20x and 50x of the laboratory of Gemology of the Group of Mineralogy and Applied Geochemistry of the Institute of Geosciences of the University Federal University of Pará (IG-UFPA) and a UNION microscope with 20x and 40x objective of the metallography laboratory of the Technical Course in Metallurgy of the Federal Institute of Education, Science and Technology of Pará (IFPA), both coupled with a camera and image system, Canon and Fujitsu, respectively, were utilized.
X-ray-Diffraction – It was of vital importance for the mineralogical characterization of most samples, formed from cryptocrystalline to microcrystalline materials. For this purpose, an X-ray diffractometer model X’PERT PRO MPD (PW 3040/60) from PANalytical, with PW3050/60 (Theta/Theta) goniometer was used. The analytical conditions comprise a copper anode X-ray tube (λCuKα1 = 1,54060Å), model PW3373 / 00, long fine focus, under conditions of 2200W, 60kv, with a Kβ Ni filter. The detector used is of type RTMS, X’Celerator. The logs were obtained in the exposure range from 4 to 75º 2θ for a total sample. The data acquisition of the records was performed with the software X’Pert Data Collector, version 2.1a, and the treatment of the data was performed with the X’Pert HighScore software version 2.1b, also of PANalytical, consulting the PDF database (Powder Diffraction File) of the ICDD (International Center for Diffraction Data). These analyses were carried out at the X-ray Diffraction Laboratory of the Geosciences Institute of the Federal University of Pará (IG-UFPA).
Infrared Spectroscopy – The technique was also used to aid in the mineralogical identification, mainly of the cryptocrystalline and / or amorphous phases, frequent in the investigated materials. The equipment used for these analyses was a Perkin-Elmer, model 1760 X FT-IR spectrophotometer, coupled to a microcomputer, with records in the range of 4000 to 400 cm -1, with measurements every 4 cm -1. Data were interpreted using Perkin-Elmer’s Graph Server software version 1.60. These analyses were also carried out in the Infrared Laboratory of the IG-UFPA
Thermal Analysis – It was used strictly, also as an auxiliary technique in the identification of Mn oxyhydroxides and in the clay minerals. Equipment was used from the laboratories of the Geowissenschaften Institut, Halle-Wittenberg Universität in Germany and IG/UFPA. A thermal analyzer, model PL Thermal Sciences with simultaneous thermal analyser STA 1000/1500 from Stanton Redcroft Ltd., with vertical cylindrical furnace and digital converter coupled to a microcomputer, was utilized. Approximately 10 mg of each sample was placed in a platinum crucible and run under an N2 flow rate of 50 cm3/min. The heating rate was 20 ° C/min, with an initial temperature of 20 ° C and final temperature of 1100 ° C.
Scanning Electron Microscopy with Dispersive Energy System (SEM/EDS) – It was also used continuously to aid in the various mineralogical and micromorphological identifications, being crucial for the characterization of the Mn and Fe oxyhydroxides and the clay minerals. Equipment from IG-UFPA, Goeldi Museum and ZWL-Lauf/Nürnberg laboratories was used. The analyses were performed using a software-controlled LEO 1450VP Scanning Electron Microscope through an X-ray dispersion spectrometry system (SED 500 DP).
Electron Probe Micro-Analyser (EPMA) – It was used to determine chemical composition mainly of oxyhydroxides of Mn (cryptotomelane), carbonates (rhodochrosite) and tentatively some fined-grains silicates (chlorite and illite). The analyses were carried out at the Institute of Physics of the Federal University of Minas, Belo Horizonte, Brazil, equipped with a JEOL electronic microprobe, model 8900RL. The electron beams (diameter of 5 μm) used during the measurements were generated from 20 kv, with a current intensity of 25 nA and integration time of 6 s.
Whole Chemical Analyses – The chemical composition of total samples and isolated fractions was determined mainly in commercial laboratories. The techniques employed were mainly fusion with metaborate and / or lithium tetraborate, followed by digestion with nitric and / or hydrochloric acid and determination by ICP-OES and ICP-MS. These analyses were performed in the ACME and ACTLab laboratories.
Geochronology – Selected samples of Mn oxyhydroxides from the sedimentary rock were subjected to the 40K/39Ar method intermediated by ACTLab and 40Ar/39Ar through the laboratories of Prof. Dr. Paulo Vasconcelos from the University of Queensland, Australia.
Mass Spectrometry – It was used for the determination of d34S in sulphides, mainly pyrite, as well as d13C in carbonaceous material, contained in sedimentary rocks mineralized in Mn oxyhydroxides. It was also used for the determination of d18O and D in kaolinite from kaolin venules that section the Mn-oxyhydroxides ore hosted by tectonic structures. These analyses were performed in ACTLab. The same technique was used to determine d13C and d18O in carbonates. Analyses were carried out at GNS in New Zealand.
Leco – It was used for the determination of organic carbon in the rock samples in the ACTLabs.
Table 1 – List of samples collected and analyzed with the respective analytical procedures carried out. DH: Drill hole; XRD: X ray diffraction; OM: optical microscope; EPMA: Electron microprobe analysis; SEM/EDS: Scanning electron microscope/Energy dispersive X spectrometry; FTIR: Fourier-transformed infrared; COM: carbonaceous organic matter; X: Indicates the respective analytical technique that was performed on each sample.
| Sample Nr. | Mine | DRILL HOLE-ID | XRD | Chemistry | OM | EPMA | SEM/EDX | K/Ar dating | δ 13C e δ 18O | δ13C PDB | δ 34S isotope | FTIR | COM |
| Azul 06 | 1 | DH167P27.30 | X | X | |||||||||
| Azul 09 | 1 | DH167P62.80 | X | X | |||||||||
| Azul 10 | 1 | DH167P68.50 | X | X | X | ||||||||
| Azul 13 | 1 | DH167P105.45 | X | X | |||||||||
| Azul 15 | 1 | DH167P107.85 | X | X | |||||||||
| Azul 18 | 1 | DH277P4.70 | X | X | |||||||||
| Azul 24 | 1 | DH277P68.25 | X | X | |||||||||
| Azul 25 | 1 | DH277P77.00 | X | X | |||||||||
| Azul 50 | B540E | X | X | ||||||||||
| Azul 81 | 1 | DH5P183.83 | X | X | |||||||||
| Azul 82 | 1 | DH5P202.00 | X | X | X | ||||||||
| Azul 83 | 1 | DH5P202.00 | X | X | X | ||||||||
| Azul 83 | 1 | DH5P216.30 | X | X | X | X | X | ||||||
| Azul 84 | 1 | DH5P220.00 | X | X | |||||||||
| Azul 88 | 1 | DH6p128.16 | X | X | X | X | |||||||
| Azul 89 | 1 | DH6P136.40 | X | X | X | X | X | ||||||
| Azul 93 | 1 | DH63P121.00 | X | X | |||||||||
| Azul 98 | 1 | DH86P63.60 | X | X | |||||||||
| Azul 102 | 1 | DH90P82.40 | X | X | |||||||||
| Azul 110 | 1 | DH174P60.40 | X | X | |||||||||
| Azul 114 | 3 | DH304P92.00 | X | X | |||||||||
| Azul 115 | 3 | DH304P94.00 | X | X | |||||||||
| Azul 120 | 1 | DH318P73.00 | X | X | X | X | |||||||
| Azul 130 | 1 | DH382 P22.60 | X | X | X | ||||||||
| Azul 140 | 1 | DH382 P73.40 | X | X | |||||||||
| Azul 142 | 1 | DH382 P 93.70 | X | X | |||||||||
| Azul 153 | 1 | DH382 P150.15 | X | X | X | ||||||||
| Azul 155 | 1 | DH382 P162.60 | X | X | X | ||||||||
| Azul 156 | 1 | DH382 P168.50 | X | X | |||||||||
| Azul158 | 1 | DH382 P176.10 | X | X | X | ||||||||
| Azul160 | 1 | DH382 P185.35 | X | X | X | ||||||||
| Azul 161 | 1 | DH382 P188.45 | X | X | X | X | |||||||
| Azul 162 | 1 | DH382P195.05 | X | X | X | X | X | X | |||||
| Azul 163 | 1 | DH382P198.25 | X | X | X | ||||||||
| Azul 164 | 1 | DH382P199.20 | X | X | X | X | X | X | |||||
| Azul 165 | 1 | DH382P202 | X | X | X | X | X | X | |||||
| Azul 166 | 1 | DH382P206.55 | X | X | X | X | X | X | |||||
| Azul 173* | 1 | DH387 P18.00 | X | X | X | ||||||||
| Azul 175* | 1 | DH387 P25.00 | X | X | |||||||||
| Azul 176* | 1 | DH387 P27.60 | X | X | X | ||||||||
| Azul 178 | 1 | DH387 P33.00 | X | X | |||||||||
| Azul 199 | 1 | DH387 P114.20 | X | X | X | X | X | ||||||
| Azul 201 | 1 | DH396P79.70 | X | X | X | X | X | ||||||
| Azul 202 | 1 | DH396P83.50 | X | X | X | ||||||||
| Azul 206 | Hand sample 4 | X | X | X | X | ||||||||
| Azul 225 | B476 pelite | X | X | X | X | ||||||||
| 1 | DH303/P69.50m-c | X | X | ||||||||||
| 1 | DH303/P69.50m-d | X | |||||||||||
| 1 | DH303/P87.55m-a | X | X | X | |||||||||
| 1 | DH303/P87.55m-f | X | X | X | |||||||||
| 1 | DH326/P57.25m-a | X | X | X | |||||||||
| 1 | DH326/P57.25m-c | X | |||||||||||
| 1 | DH326/P57.25m-d | X | X | ||||||||||
| 1 | DH348/P33.80 | X | X | ||||||||||
| 1 | DH560 | X | X | ||||||||||
| DH728/P2.66m-a | X | X | |||||||||||
| DH728/P12.35m-d | X | X | |||||||||||
| MF-14 | X | X | |||||||||||
| MF-22 | X | X | |||||||||||
| MF-24 | X | X | |||||||||||
| MN1-8 | 1 | X | X | ||||||||||
| MN1-22 | 1 | X | X | ||||||||||
| MN1-25A | 1 | X | X | ||||||||||
| MN1-29A | 1 | X | X | X | X | ||||||||
| MN1-30 | 1 | X | X | X | |||||||||
| MN1-33 | 1 | X | X | ||||||||||
| MUGEO2460 | Carrapato | X | X | X | X | ||||||||
| ESTMn01 | Carrapato | X | X | X | X | ||||||||
| MF-42 | X | ||||||||||||
| MN1-31 | 1 | X | |||||||||||
| MF-24 | X | ||||||||||||
| MF-19 | X | ||||||||||||
| MF-10 | X | ||||||||||||
| MF-17 | X | ||||||||||||
| MN1-24 | 1 | X | X | X | |||||||||
| MF-23 | X | ||||||||||||
| MF-21 | X | ||||||||||||
| MN1-1 | 1 | X | X | ||||||||||
| MN1-27 | 1 | X | X | ||||||||||
| MN1-32 | 1 | X | X | ||||||||||
| MN1-35 | 1 | X | X | ||||||||||
| MN3-1 | 3 | X | X | ||||||||||
| MN3-2 | 3 | X | X | X | |||||||||
| MN3-3 | 3 | X | X | X | |||||||||
| MN3-7 | 3 | X | X | X | |||||||||
| MN1-18 | 1 | X | X | ||||||||||
| MN1-10 | 1 | X | X | ||||||||||
| MN1-31 | 1 | X | X | ||||||||||
| MN1-24 | 1 | X | X |
RESULTS
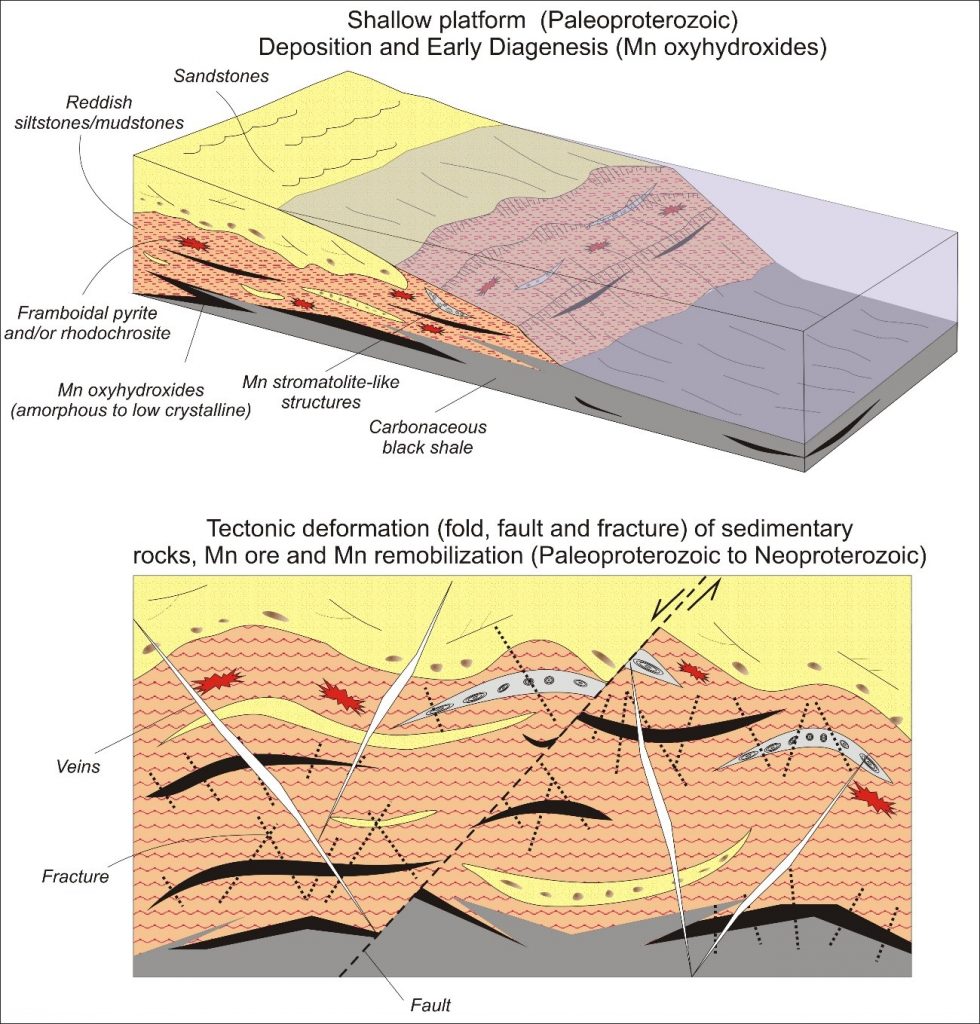
Ore Geology – The outcrop and subsurface (approximately up to 50 m deep) manganese ore expressed by Mn-oxyhydroxides in the mines of Azul were mined in the first 16 years (1984 through 2000). From the 2000s onwards, the dominant ore was at a higher depth. The former was considered to be supergenic (lateritic), and the other was primary (Costa et al., 2005). The outcropped and subsurface mineralized materials, manganese ores, involve ferrous-manganesiferous, and manganesiferous crusts and ferrous-aluminum-manganesiferous pisolites and fragments (Figures 4 through 7). Underlying these materials or even outcropping in the lower part or border of the mines is the manganese-bearing or manganese-free black shales and siltstones/mudstones and sandstones (Figures 4 through 7). Supergenic ores were part of the full to erosional truncated lateritic profile. On the other side is the manganese oxyhydroxide ore mined in the last 16 years in the Azul deposit hosted by dark grey to black shales, finely laminated, which forms lenses up to 4 m thick and a hundred metres in length. They are hosted by thick reddish mudstones/siltstones and sandstones, sometimes striped (Figures7 and 8 C), or micro faulted (Figure 8 C). The contact between these rocks and the shales is gradational (Figures 7 and 8 A and B), in which the Mn oxyhydroxides give rise to dark grey carbonaceous material (Figure 8 A, B and D) forming the grey siltstones/mudstones. In addition to the Mn oxyhydroxides, mainly cryptomelane and birnessite, these rocks are formed by illite/sericite, chlorite, kaolinite, and quartz (Table 2) and, as appropriate, fine laminated carbonaceous material (Figure 8 A and B). The red siltstones, formed by quartz, kaolinite, illite and microcrystalline haematite, may contain centimetre to decimetre large nodules of Mn oxyhydroxides, or millimetre to centimetre stratified to laminated lenses of those same materials (Figure 8 C). These lenses become more and more frequent as they approach the zone of contact with the greasy grey shales (Figure 8 D).

Figure 7 – General view of the open pit of Mine 1, at Azul manganese deposit in Carajás showing the domain of reddish mudstones/siltstones/sandstones, which host the distinct lenses of carbonaceous and manganese black shales/ores. On the top stands the lateritic manganese ore.

Figure 8 – Some geological aspects of manganese ore and country rocks in the Mine 1 of Azul: (A) Contact zone between Mn-bearing black shale on the left side, geay carbonaceous shale in the center and reddish siltstone on the right side of the image; (B) Detail of the contact between dark grey to black Mn-bearing shale (Mn ore) on the upper part of the left side of the image and carbonaceous grey shale underlying it; (C) Banded Mn-hosting rocks represented by reddish mudstones intercalated with carbonaceous grey shales and fine kaolin laminae and flakes; (D) Detail of the stratified to massive ore formed by Mn-oxyhydroxides grading downward to fine stratified Mn-rich black shales intercalated with dark grey shales; flakes of kaolin can be also observed.
Table 2 – The minerals identified and their general evolution in the manganese deposits of Azul, Carajás Mineral Province. (?): The information needs further investigation to be fully confirmed.
| Minerals | Environmental processes | |||
| Syngenetic
(sedimentary) |
Early diagenetic
(anchimetamorphic) |
Epigenetic
(tectonic) |
Supergene (lateritic) after Costa et al. (2005) | |
| Illite | ||||
| Sericite | ||||
| Kaolinite | ||||
| Chlorite | ||||
| Smectite | ||||
| K-Feldspar | ||||
| Quartz | ||||
| Pyrite | ||||
| Chalcopyrite | ||||
| Amorphous Mn- oxyhydroxides | ||||
| Rhodochrosite | ||||
| Carbonaceous organic matter | ||||
| Cryptomelane | ||||
| Cryptomelane-hollandite | ||||
| Coronadite | ||||
| Birnessite | ? | |||
| Hollandite | ||||
| Pyrolusite | ||||
| Nsutite | ||||
| Todorokite | ||||
| Lithiophorite | ||||
| Gibbsite | ||||
| Goethite | ||||
| Hematite | ||||
This sedimentary succession, which reaches approximately 200 m in thickness in mine 1 (Figure 5), is correlated with the Águas Claras of Araújo et al., 1988; and Nogueira et al., 1995. Araújo and Sousa (2018) proposed that the maximum age for mineralization would be approximately 2,609 Ma from U-Pb dating of detrital zircons. These minerals were extracted from rocks of the Águas Claras formation within the Azul mine and the data confirm the Neoarchean age (Costa et al., 1995). Previous studies show, however, that the sediments of the Águas Claras Formation pointed out by indirect geochronological data have a maximum age of 1.8 Ga (Beauvais et al., 1987). Our t five geochronological analyses performed (Table 3) showed ages compatible with those presented in the previous one, with three of them approximately 2.1 Ga.
Table 3 – 40K/39Ar geochronologic ages get in red siltstones hosting the sedimentary manganese ore (dark gray to black shales) in the Azul deposits.
| Sample ID: | 40K/39Ar Ages |
| MN1-8 | 2359±62 |
| MN1-22 | 2006±51 |
| MN1-25A | 2044±53 |
| MN1-29A | 1792±47 |
| MN1-30 | 2058±53 |
The grey colour intensifies with the increase of the content of carbonaceous organic matter (COM), while black is correlated with the Mn-oxyhydroxides (Figures 7 and 8). The transition from red siltstones/mudstones to black shales occurs both laterally and at the top and bottom contacts (Figures 7 and 8 A and B). Dark siltstones and black shales behave as real lenses, mainly from the intermediate zone to the bottom of mine 1, having a hundred metres of apparent length and a maximum thickness of 15 m on the mining fronts (Figure 7). The geological section presented by Bernardelli and Beisiegel (1978) and modified by Beauvais et al. (1987) already foresee the mineralization associated with the mega-lenses body form (Figure 2).
Most of the mega-lenses exposed during manganese ore mining in 2009, 180 m below the top of the plateau surface, that is, at the topographic level of 400 m, consisted of Mn-oxy-hydroxides (cryptomelane), with a massive to laminar aspect, with kaolin blades, venules and sulphide pockets, occurring within a pack of black shales grading to dominant striped or striped, red siltstones (Figures 7 through 9 C). This mode of occurrence suggests that the Mn-oxyhydroxides of the mega-lens of black shale are contemporaneous with sedimentation or early diagenesis (Table 2). In the Azul mines, this material is special due to its high content, and it is classified as dioxide, dominated by cryptomelane. Similar lenses extend through almost all mines at different vertical levels and horizontal positions hosted by reddish siltstones to sandstones that extend along all the mine´s area (Figures 6 and 7). Grey siltstones/mudstones and grey to black shales, with carbonaceous organic matter (COM), contain kaolin (kaolinite, quartz and illite) in fine layers parallel to the bedding or cross cutting the same (Figure 8), generally a characteristic feature of them. They can also present venules and nodules/concretions or framboids of pyrites, sometimes in cubic crystals and/or chalcopyrite masses, and even gold. The distribution of the COM is concordant with the bedding, and gives the grey colour, fissiliness and greasiness to the sediments. They can be well correlated to black shales present in global manganese sedimentary mineralizations, already proposed by Beauvais (1984).
In contrast to the pioneering work in the Azul deposits, which began with drill hole 5, which allowed for identification of primary manganese units constituted by Mn carbonate (Bernadelli & Beisiegel, 1978 illustrated in Figure 2; Valarelli et al., 1978), the data obtained in this work during several field works, and deep drill holes did not find these rocks. The geological observations in the mine in recent years when it reached greater depths and therefore fresh rocks, in addition to more recent deep drill holes (up to 200m in depth), the depth of carbonates in drill hole 5, show that the previous considered proto-ore (the marls: carbonate-bearing shales, mainly rhodochrosite) were no longer found. This sequence described here in the lower zone of the actual pits and in the deepest drilling holes does not present any evidence of origin of these oxyhydroxides from the chemical weathering of rhodochrosite, which is always restricted to millimeter laminae and mostly veinlets.
Deformation-related Mineralization – The sedimentary packet (red siltstones/mudstones, sandstones, and the manganese, mineralized or not, black shales in the mines of Azul) was intensely tectonically deformed. The set as a whole is folded, with main manganese ore from central mine 1 occupying the upper portion of the east-west anticline (Bernardelli & Beisiegel, 1978) (Figures 5 through 7). The folding was more intense and tighter at the Carrapato and Mine 3 (Figure 9 A and B). North-south and northwest-southeast faults and shear zones overprinted the entire sequence (Araujo & Sousa, 2018). The tectonic deformation also developed intense cracking, micro faults, folds and micro folds (Figures 9 C and D; 10 A through E). At the intersections between failures and fractures developed massive to colloform, locally druse form, very dense, sometimes cavernous, ore bodies, made of pyrolusite, manganite, and perhaps nsutite (Figure 10 C). These minerals were not found in the dark grey to black shales and non-deformed siltstones. In the shear zones (mine 3) (Figure 9), these manifestations extend for tens of metres in length. Here the manganese oxyhydroxides are massive to crystalline and form rich ore. The hinges of the tight folds served as trapping for Mn-oxyhydroxides accumulation in high contents (Figure 10 A through C), besides the pockets. Millimetric to centimetric veinlets fulfilled with kaolin and Mn-oxyhydroxides (Figure 10 E), as well as (Fe,Cu)-sulphides have been formed.
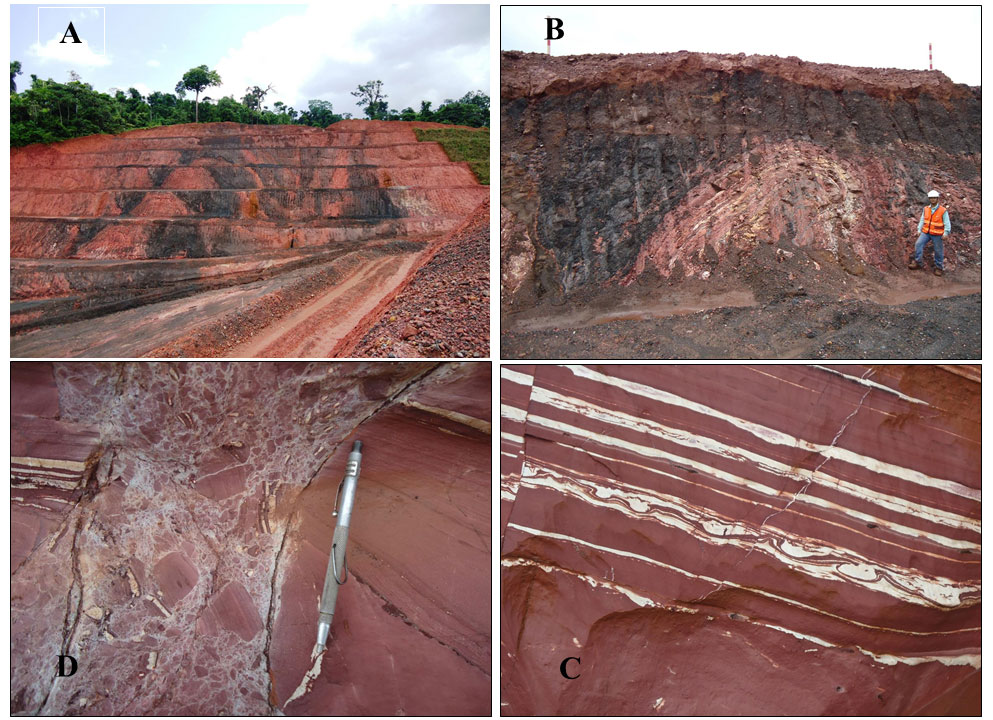
Figure 9 – Some tectonic features observed in the Mine 3 in Azul: (A) Mn-ore and country rocks hosted by tight folded and faulted. On the right side one can see swarms of kaolin venules; (B) A typical fold established on the reddish siltstones, kaolin and the Mn-oxyhydroxide layers; (C) A typical stripped mudstones slight faulted, showing convoluted folds between strata and kaolin venules; (D) Tectonic breccia developed in fault zone inside of the reddish sandstones, being the rock fragments involved by kaolin and crosscut by kaolin venules.

Figure 10 – Further new tectonic features observed in the Mine 3 in Azul showing concentration of Mn-oxyhydroxide ore: (A) Tight folding close to shear zones, in which pockets of high grade Mn-ore have been formed;( B) A detail of the shear zone with Mn-oxyhydroxides, quartz and pervasive kaolin veinlets; (C) Aggregates of acicular overgrowth of pyrolusite and manganite in the shear zone; (D) Slight stratified massive Mn-oxyhydroxides ore with sandstone laminae, faulted and cross cut by massive Mn-oxide veinlets; (E) Siltstones intensively cross cut or invaded over the bedding plans (small pockets) by Mn-oxides and kaolin veinlets.
Micromorphology and Mineralogy – Because the present work emphasizes the sedimentary sequence and its diagenetic and tectonic alteration, greater emphasis will be given only to the minerals and their micromorphological aspects that make up these materials. Those related to lateritic cover will only be mentioned. The sedimentary rocks mineralized in Mn-oxyhydroxides (black shales and dark grey and black siltstones) and their hosting rocks (red siltstones/mudstones and striped, red sandstones) are formed by quartz, illite/sericite, chlorite, and kaolinite and are distinguished by concentrations of Mn-oxyhydroxides, carbonaceous organic material (COM) and haematite (Table 2). COM is typical of the lenses of grey shales and in the transition zone between manganese-rich shales (Mn ore) and red siltstones/sandstones, and it is represented by very fine grey material composed mostly of amorphous carbon. The Mn-oxyhydroxides are the main minerals of black siltstones and shales and in part of the transitional zone. They are represented by cryptomelane and cryptomelane-hollandite (Figure 11), the most typical manganese mineral of the sedimentary sequence, displaying distinct micromorphological aspects (Figure 12) that are birnessite restricted (Table 2). Haematite is characteristic of siltstones and red sandstones, striped or not, and is microcrystalline and finely disseminated in these rocks. Pyrite, chalcopyrite and rhodochrosite occur discreetly, usually as venules and microbands concordant with stratification. Locally, they were identified by SEM/EDS Ti oxide needles, which by the mode of occurrence may correspond to rutile.
In the pockets, faulting, and shear zones, and even in the hinges of the mega-folds, cryptomelane persists, however pyrolusite is more abundant, besides the nsutite. Pyrolusite occurs in mm to cm crystals in typical druse intergrowths. Therefore, this mineral is a guide to the deformed zones in the Azul mine and at the same time constitutes locally rich concentrations of Mn. The kaolin veins and venules are formed obviously by kaolinite (Figure 10 E), which may contain quartz, pyrite, chalcopyrite and sometimes rhodochrosite. The kaolinite in these venules and bands shows well-developed micrometric pseudo-hexagonal crystals. Quartz tends to be milky to microcrystalline, rarely macrocrystalline. The rhodochrosite forms micrometric mass, milky white, slightly transparent and with grey shade occurring together with quartz and pyrite in a micritic texture, as well as, in veinlets in the black shales. In the quartz venules that intercept the micritic masses with rhodochrosite, this mineral generally borders the quartz venular body.
Microprobe analyses (Table 4) of the rhodochrosite masses show important contents of Fe (FeO), 4.84 to 5.88%, while Mg (MgO) contents ranged from 0.02 to 0.36 and Ca (CaO) from 0.02 to 0.93, suggesting the possible presence of kutnohorite, and / or ankerite and siderite, or even calcite, found sporadically in venules.
The AZUL-164 sample was one of the few samples of black shales with micritic rhodochrosite ranging from main mineral to accessory, alongside quartz, illite, chlorite and pyrite. Calcite is an accessory. This mineralogical composition can be deduced from the results of chemical analyses by microprobe at different points in this sample (Table 5).
Table 4 – EPMA chemical composition of rhodochrosite from Mn-bearing shales.
| Rhodochrosites | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Theoretic |
| Na2O | nd | nd | nd | nd | nd | 0.02 | nd | nd | nd | nd | |
| SiO2 | 0.02 | nd | 0.03 | 0.02 | 0.03 | 0.05 | nd | 0.02 | 0.04 | 0.06 | |
| Al2O3 | 0.04 | 0.03 | nd | 0.02 | nd | 0.06 | 0.01 | 0.01 | 0.04 | 0.04 | |
| MgO | 0.11 | 0.20 | 0.22 | 0.03 | 0.02 | 0.10 | 0.11 | 0.26 | 0.36 | 0.05 | |
| K2O | nd | nd | nd | nd | 0.02 | 0.01 | 0.03 | nd | 0.01 | 0.01 | |
| MnO | 52.77 | 52.46 | 53.12 | 52.50 | 52.73 | 52.66 | 52.91 | 51.70 | 51.29 | 53.08 | 61.71 |
| PbO2 | nd | nd | 0.08 | 0.04 | 0.04 | 0.19 | nd | nd | nd | nd | |
| CaO | 0.38 | 0.87 | 0.66 | 0.14 | 0.02 | 0.11 | 0.32 | 0.81 | 0.93 | 0.08 | |
| TiO2 | 0.03 | 0.02 | nd | nd | nd | nd | nd | nd | nd | 0.03 | |
| SO3 | 0.04 | nd | 0.03 | 0.03 | nd | 0.01 | 0.03 | 0.07 | 0.02 | 0.05 | |
| Cl | nd | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | Nd | |
| FeO | 5.17 | 5.15 | 5.36 | 5.18 | 5.30 | 4.84 | 4.91 | 5.47 | 5.88 | 5.42 | |
| BaO | nd | nd | 0.17 | nd | nd | nd | nd | nd | nd | 0.09 | |
| Total | 59.80 | 60.00 | 60.95 | 59.20 | 59.42 | 59.29 | 59.57 | 59.60 | 59.84 | 60.15 |
In addition, these analyses allow for the identification of microcrystalline intergrowth between illite and chlorite and make it possible to delimit chlorite domains (Table 6).
Table 5 – EPMA chemical analyses of the manganese-bearing shales with microcrystalline to micritic rhodochrosite and calcite, and chlorite, illite, pyrite and quartz.
| Samples/Mineralogy/
Main oxide elements |
Na2O | SiO2 | Al2O3 | MgO | K2O | MnO | PbO2 | CaO | TiO2 | SO3 | Cl | FeO | BaO | H2O | Total |
| AZUL164-C2A,
chlorite, illite, rhodochrosite, pyrite, calcite |
0.05 | 29.07 | 16.45 | 14.95 | 0.98 | 6.93 | nd | 0.86 | 0.05 | 4.96 | 0.02 | 6.02 | nd | 3.32 | 83.66 |
| AZUL164-I,
chlorite, illite, rhodochrosite, pyrite, calcite |
0.05 | 38.49 | 15.89 | 12.94 | 1.34 | 6.82 | nd | 1.27 | 0.07 | 3.16 | 0.04 | 6.41 | 0.11 | 3.61 | 90.20 |
| AZUL164-D,
chlorite, illite, rhodochrosite, quartz, pyrite |
0.05 | 33.82 | 16.46 | 13.56 | 3.53 | 6.36 | nd | 0.90 | 0.10 | 2.88 | 0.03 | 5.56 | nd | 3.41 | 86.66 |
| AZUL164-B,
rhodochrosite, illite, calcite, quartz, pyrite |
0.04 | 20.89 | 8.18 | 0.63 | 3.80 | 18.20 | nd | 2.78 | 0.28 | 2.03 | 0.06 | 1.10 | nd | 2.08 | 60.06 |
| AZUL164-E,
chlorite, rhodochrosite, (illite), quartz, pyrite, calcite |
0.07 | 26.73 | 11.83 | 8.59 | 0.52 | 12.50 | nd | 4.74 | 0.03 | 4.03 | 0.03 | 6.09 | 0.04 | 2.93 | 78.12 |
| AZUL164-C2B,
quartz, chlorite, rhodochrosite |
0.04 | 53.68 | 10.73 | 8.84 | 0.10 | 4.32 | nd | 0.04 | 0.05 | 0.26 | 0.02 | 4.60 | nd | 3.69 | 86.36 |
| AZUL164-A,
chlorite, rhodochrosite, illite, pyrite |
0.03 | 32.15 | 12.77 | 12.87 | 3.19 | 3.77 | nd | 0.75 | 0.13 | 0.83 | 0.02 | 2.14 | 0.13 | 2.89 | 71.65 |
| AZUL164-C,
quartz, chlorite(?), rhodochrosite |
0.04 | 82.72 | 2.18 | 2.63 | 0.02 | 2.49 | 0.04 | 1.69 | nd | 0.17 | 0.02 | 0.97 | 0.09 | 4.45 | 97.52 |
Table 6 – EPMA chemical analyses of illite + chlorite, and chlorite and in grey shales.
| Analyszed points | 1 | 2 | 3 | 4 | 5 | 5 | Webmineral* | |
| Illite + chlorite (?) | Illte + chlorite (?) | Chlorite
(?)+ |
Chlorite
(?) + |
Chlorite
(?) + |
Chlorite | Chlorite | ||
| Na2O | 0.01 | 0.01 | 0.05 | 0.03 | 0.00 | 0.01 | ||
| SiO2 | 39.87 | 38.23 | 29.07 | 32.15 | 31.54 | 30.17 | 30.28 | |
| Al2O3 | 18.76 | 18.24 | 16.45 | 12.77 | 17.13 | 17.81 | 17.13 | |
| MgO | 19.49 | 18.86 | 14.95 | 12.87 | 19.62 | 25.22 | 25.39 | |
| K2O | 7.90 | 7.93 | 0.98 | 3.19 | 0.00 | 0.32 | ||
| MnOa | 2.28 | 2.54 | 6.93 | 3.77 | 0.00 | 7.29 | ||
| PbO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| CaO | 0.08 | 0.01 | 0.86 | 0.75 | 0.00 | 0.02 | ||
| TiO2 | 0.44 | 0.44 | 0.05 | 0.13 | 0.00 | 0.04 | ||
| SO3 | 0.01 | 0.07 | 4.96 | 0.83 | 0.00 | 0.02 | ||
| Cl | 0.00 | 0.04 | 0.02 | 0.02 | 0.00 | 0.01 | ||
| FeOb | 4.80 | 4.69 | 6.02 | 2.14 | 8.17 | 5.51 | 15.09 | |
| BaO | 0.00 | 0.00 | 0.00 | 0.13 | 0.12 | 0.04 | ||
| H2O | 3.83 | 3.71 | 3.32 | 2.89 | 3.24 | 3.51 | 12.11 | |
| Total | 97.47 | 94.75 | 83.66 | 71.65 | 79.83 | 89.96 | ||
aTotal Mn as MnO; b Total Fe as FeO. *Webmineral, http://www.webmineral.com/data/Clinochlore.shtml#.XnjQBIhKjIU, accessed March 23, 2020.
Cryptomelane and cryptomelane-hollandite, the main mineral of Mn in the Azul deposits, presents in masses that are finely stratified or still of massive aspect (Figure 12). In the sedimentary package of Azul the cryptomelane occurs associated with illite/sericite, sometimes quartz, kaolinite, pyrite and COM (Table 2). Its chemical composition changes in terms of the most common members of the group, K, Ba, Sr and Pb, where the major highlight is for Ba, locally represented by the cryptomelane-hollandite series (Tables 7 and 8), generally associated with both discordant and concordant venules (Figure 12). Cryptomelane also appears as an ore mineral in the venules and filling pockets and fissures. However, no differences in its chemical composition were observed between them due to these different modes of occurrences. In turn, they are cryptomelane-hollandites poor in SrO (Table 8), and coronadite (Pb) was identified even more restrictively by both XRD and EPMA. The EPMA chemical composition of cryptomelane-hollandites from Azul, when compared to those other deposits, diverge in contents of Al, Fe, Ni, Co, Cu and Zn. (Table 9), generally higher.
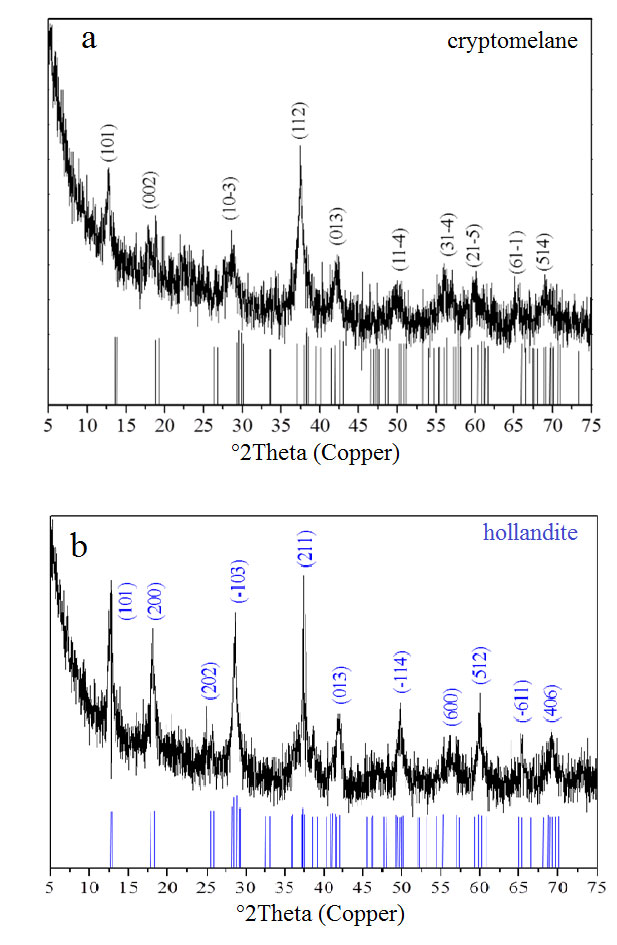
Figure 11 – XRD analyses for the most common Mn-oxyhydroxides ore in Azul: a) typical domain of cryptomelane; b) and typical domain of cryptomelane-hollandite.
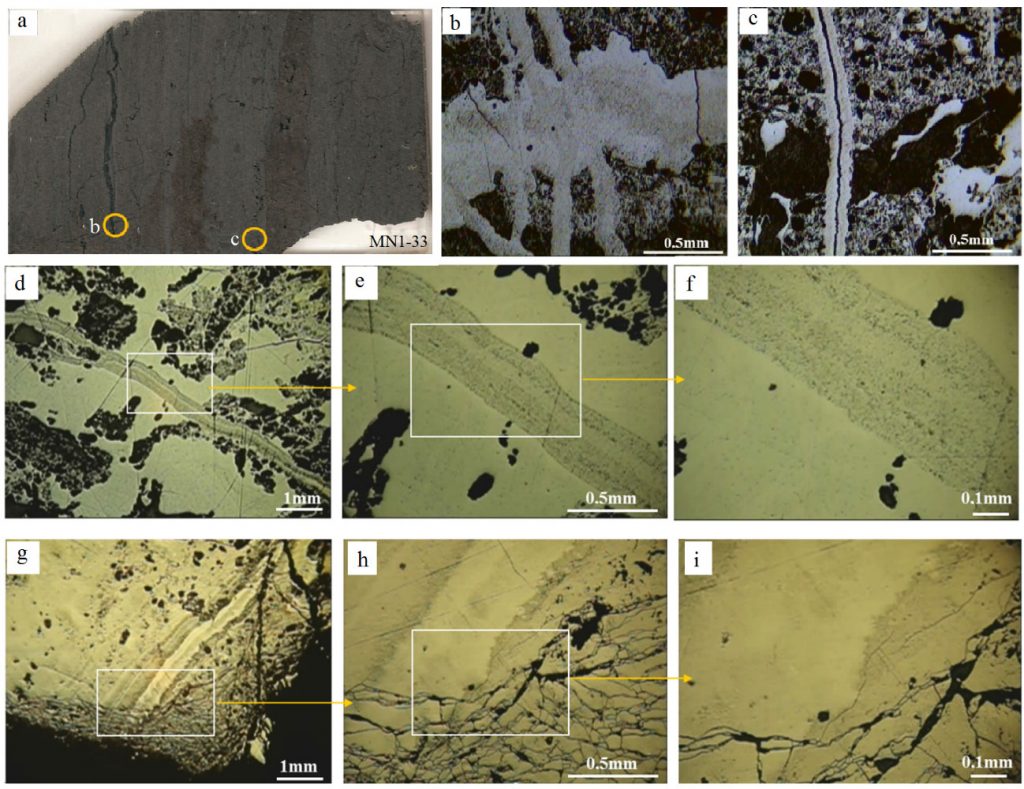
Figure 12 – Some optical microscope features of the Mn-oxyhydroxides in Azul deposits: (a) General view of banded massive manganese oxyhydroxides (sample MN1-33 ), mainly cryptomelane cross cut by cryptomelane veinlets; (b) Cryptomelane veinlets in polished thin sections, sample MN1-33; (c) cryptomelane veinlet discordant to bedding, polished thin section; (d) massive and veinlet of cryptomelane; (e) Detail of late cryptomelane veinlets; (f) Detail of previous image showing the typical banding; (g) bands and veinlets of late cryptomelanes; (h) Detail of the previous image with breccia-like feature; (i) Detail of the previous image. Optical microscope images, reflecting light.
Table 7 – EPMA chemical composition (oxide Wt.%) and calculated chemical formulas for cryptomelane, considering 16 oxygens pro formula.
| Point analysed | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| SiO2 | 0.89 | 0.78 | 0.93 | 0.35 | 0.37 | 0.72 | 0.91 | 0.41 | 0.85 | 0.90 |
| Al2O3 | 7.40 | 6.90 | 7.71 | 5.40 | 5.56 | 7.23 | 8.76 | 4.69 | 7.46 | 8.14 |
| FeO | 5.80 | 0.60 | 1.67 | 2.53 | 3.89 | 3.57 | 1.99 | 2.30 | 1.12 | 1.49 |
| MnO2 | 84.42 | 87.03 | 86.03 | 84.27 | 82.01 | 83.74 | 77.98 | 86.64 | 84.72 | 78.99 |
| MgO | 0.02 | 0.00 | 0.16 | 0.29 | 0.35 | 0.46 | 0.14 | 0.23 | 0.06 | 0.15 |
| CaO | 0.08 | 0.04 | 0.08 | 0.20 | 0.17 | 0.24 | 0.10 | 0.18 | 0.07 | 0.11 |
| Na2O | 0.08 | 0.10 | 0.10 | 0.15 | 0.08 | 0.10 | 0.18 | 0.12 | 0.11 | 0.12 |
| K2O | 2.73 | 4.09 | 3.49 | 2.07 | 1.96 | 2.47 | 3.08 | 2.54 | 4.35 | 3.05 |
| TiO2 | 0.13 | 0.02 | 0.03 | 0.04 | 0.06 | 0.11 | 0.04 | 0.11 | 0.06 | 0.02 |
| P2O5 | 0.15 | 0.11 | 0.11 | 0.11 | 0.12 | 0.21 | 0.20 | 0.17 | 0.10 | 0.12 |
| SO3 | 0.02 | 0.01 | 0.00 | 0.05 | 0.08 | 0.05 | 0.10 | 0.03 | 0.01 | 0.07 |
| V2O3 | 0.21 | 0.00 | 0.08 | 0.00 | 0.00 | 0.00 | 0.15 | 0.02 | 0.02 | 0.00 |
| CoO | 0.08 | 0.11 | 0.13 | 0.16 | 0.16 | 0.20 | 0.13 | 0.17 | 0.15 | 0.13 |
| NiO | 0.03 | 0.00 | 0.07 | 0.08 | 0.07 | 0.19 | 0.03 | 0.08 | 0.00 | 0.03 |
| CuO | 0.24 | 0.30 | 0.21 | 0.24 | 0.22 | 0.25 | 0.24 | 0.23 | 0.31 | 0.26 |
| ZnO | 0.06 | 0.14 | 0.18 | 0.06 | 0.02 | 0.11 | 0.15 | 0.09 | 0.16 | 0.11 |
| As2O5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SrO | 0.07 | 0.06 | 0.04 | 0.06 | 0.04 | 0.06 | 0.07 | 0.07 | 0.05 | 0.02 |
| ZrO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 |
| BaO | 0.20 | 0.00 | 0.02 | 0.03 | 0.00 | 0.06 | 0.02 | 0.04 | 0.00 | 0.05 |
| PbO | 0.07 | 0.03 | 0.03 | 0.05 | 0.00 | 0.00 | 0.01 | 0.22 | 0.00 | 0.02 |
| Total | 98.68 | 100.31 | 101.06 | 94.14 | 95.17 | 99.75 | 94.28 | 98.39 | 99.57 | 93.75 |
| B cations | ||||||||||
| Al3+ | 1.041 | 0.943 | 1.043 | 0.772 | 0.806 | 0.996 | 1.265 | 0.659 | 1.028 | 1.181 |
| Fe2+ | 0.579 | 0.059 | 0.161 | 0.257 | 0.400 | 0.349 | 0.204 | 0.229 | 0.109 | 0.154 |
| Mn4+ | 6.633 | 6.972 | 6.829 | 7.068 | 6.969 | 6.763 | 6.604 | 7.134 | 6.849 | 6.725 |
| Mg2+ | 0.003 | 0.000 | 0.028 | 0.052 | 0.064 | 0.079 | 0.025 | 0.040 | 0.010 | 0.027 |
| Co2+ | 0.008 | 0.010 | 0.012 | 0.016 | 0.016 | 0.019 | 0.012 | 0.016 | 0.014 | 0.013 |
| Ni2+ | 0.002 | 0.000 | 0.006 | 0.008 | 0.007 | 0.018 | 0.003 | 0.007 | 0.000 | 0.002 |
| Cu2+ | 0.022 | 0.026 | 0.018 | 0.022 | 0.021 | 0.022 | 0.022 | 0.021 | 0.028 | 0.024 |
| Zn2+ | 0.005 | 0.012 | 0.015 | 0.005 | 0.002 | 0.009 | 0.014 | 0.007 | 0.014 | 0.010 |
| Total | 8.294 | 8.021 | 8.113 | 8.200 | 8.284 | 8.254 | 8.150 | 8.114 | 8.055 | 8.137 |
| A cations | ||||||||||
| Ca2+ | 0.010 | 0.005 | 0.010 | 0.025 | 0.023 | 0.030 | 0.013 | 0.023 | 0.009 | 0.015 |
| Na+ | 0.019 | 0.022 | 0.022 | 0.035 | 0.020 | 0.022 | 0.043 | 0.029 | 0.024 | 0.029 |
| K+ | 0.416 | 0.605 | 0.511 | 0.321 | 0.308 | 0.368 | 0.482 | 0.385 | 0.650 | 0.479 |
| Sr2+ | 0.005 | 0.004 | 0.002 | 0.004 | 0.002 | 0.004 | 0.005 | 0.005 | 0.003 | 0.001 |
| Ba2+ | 0.009 | 0.000 | 0.001 | 0.002 | 0.000 | 0.003 | 0.001 | 0.002 | 0.000 | 0.002 |
| Pb2+ | 0.002 | 0.001 | 0.001 | 0.002 | 0.000 | 0.000 | 0.000 | 0.007 | 0.000 | 0.000 |
| Total | 0.461 | 0.636 | 0.547 | 0.389 | 0.353 | 0.427 | 0.544 | 0.451 | 0.686 | 0.527 |
Table 8 – EPMA chemical composition (Wt.%) and calculated chemical formulas for the series cryptomelane-hollandite, considering 16 oxygens pro formula.
| Point analyzed | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| SiO2 | 0.18 | 0.21 | 0.28 | 0.22 | 0.12 | 0.38 | 0.14 | 0.35 | 1.28 |
| Al2O3 | 4.36 | 4.57 | 6.53 | 5.27 | 2.26 | 5.61 | 3.15 | 5.97 | 7.43 |
| FeO | 3.51 | 3.43 | 0.05 | 0.07 | 0.03 | 0.15 | 0.21 | 1.16 | 4.45 |
| MnO2 | 85.53 | 82.71 | 83.56 | 83.26 | 90.71 | 81.92 | 87.70 | 84.47 | 70.07 |
| MgO | 0.01 | 0.00 | 0.02 | 0.01 | 0.05 | 0.06 | 0.10 | 0.14 | 0.05 |
| CaO | 0.03 | 0.05 | 0.01 | 0.05 | 0.15 | 0.05 | 0.13 | 0.12 | 0.17 |
| Na2O | 0.00 | 0.07 | 0.05 | 0.06 | 0.10 | 0.00 | 0.00 | 0.04 | 0.04 |
| K2O | 3.06 | 2.96 | 3.94 | 3.76 | 5.14 | 3.90 | 3.26 | 3.78 | 3.09 |
| TiO2 | 0.16 | 0.14 | 0.05 | 0.06 | 0.02 | 0.07 | 0.06 | 0.09 | 0.26 |
| P2O5 | 0.52 | 0.45 | 0.81 | 0.66 | 0.65 | 0.89 | 0.27 | 0.91 | 1.27 |
| SO3 | 0.02 | 0.04 | 0.00 | 0.01 | 0.00 | 0.03 | 0.06 | 0.11 | 0.28 |
| V2O3 | 0.25 | 0.33 | 0.63 | 0.33 | 0.40 | 0.48 | 0.10 | 0.44 | 0.82 |
| CoO | 0.09 | 0.13 | 0.53 | 0.83 | 0.21 | 0.70 | 0.28 | 0.11 | 0.53 |
| NiO | 0.00 | 0.03 | 0.03 | 0.08 | 0.05 | 0.10 | 0.09 | 0.12 | 0.00 |
| CuO | 0.55 | 0.56 | 1.00 | 1.37 | 1.41 | 1.44 | 0.84 | 0.68 | 0.81 |
| ZnO | 0.00 | 0.05 | 0.02 | 0.05 | 0.10 | 0.08 | 0.01 | 0.14 | 0.03 |
| As2O5 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SrO | 0.00 | 0.00 | 0.04 | 0.00 | 0.02 | 0.00 | 0.01 | 0.01 | 0.00 |
| ZrO2 | 0.00 | 0.00 | 0.00 | 0.09 | 0.05 | 0.04 | 0.00 | 0.00 | 0.01 |
| BaO | 2.49 | 2.65 | 1.21 | 1.35 | 0.34 | 1.71 | 1.56 | 2.00 | 2.11 |
| PbO | 0.32 | 0.28 | 0.08 | 0.00 | 0.02 | 0.00 | 0.06 | 0.00 | 0.04 |
| Total | 101.08 | 98.76 | 98.82 | 97.52 | 101.84 | 97.62 | 98.01 | 100.64 | 92.74 |
| B cations | |||||||||
| Al3+ | 0.612 | 0.657 | 0.915 | 0.754 | 0.312 | 0.803 | 0.450 | 0.827 | 1.120 |
| Fe2+ | 0.349 | 0.350 | 0.005 | 0.007 | 0.003 | 0.015 | 0.02 1 | 0.114 | 0.476 |
| Mn4+ | 7.034 | 6.971 | 6.869 | 6.989 | 7.337 | 6.872 | 7.342 | 6.859 | 6.192 |
| Mg2+ | 0.001 | 0.000 | 0.004 | 0.002 | 0.009 | 0.011 | 0.017 | 0.024 | 0.010 |
| Co2+ | 0.008 | 0.013 | 0.05 1 | 0.081 | 0.019 | 0.068 | 0.027 | 0.010 | 0.055 |
| Ni2+ | 0.000 | 0.003 | 0.003 | 0.008 | 0.005 | 0.010 | 0.009 | 0.012 | 0.000 |
| Cu2+ | 0.050 | 0.052 | 0.089 | 0.125 | 0.124 | 0.132 | 0.077 | 0.060 | 0.079 |
| Zn2+ | 0.000 | 0.004 | 0.001 | 0.005 | 0.009 | 0.007 | 0.001 | 0.012 | 0.003 |
| Total | 8.054 | 8.050 | 7.938 | 7.969 | 7.819 | 7.919 | 7.944 | 7.917 | 7.933 |
| C cations | |||||||||
| Ca2+ | 0.003 | 0.007 | 0.001 | 0.007 | 0.018 | 0.006 | 0.017 | 0.015 | 0.023 |
| Na+ | 0.000 | 0.016 | 0.010 | 0.015 | 0.024 | 0.000 | 0.000 | 0.008 | 0.010 |
| K+ | 0.464 | 0.461 | 0.598 | 0.583 | 0.767 | 0.605 | 0.504 | 0.567 | 0.504 |
| Sr2+ | 0.000 | 0.000 | 0.002 | 0.000 | 0.002 | 0.000 | 0.000 | 0.001 | 0.000 |
| Ba2+ | 0.116 | 0.126 | 0.057 | 0.064 | 0.016 | 0.081 | 0.074 | 0.092 | 0.106 |
| Pb2+ | 0.010 | 0.009 | 0.003 | 0.000 | 0.001 | 0.000 | 0.002 | 0.000 | 0.001 |
| Total | 0.594 | 0.619 | 0.670 | 0.669 | 0.827 | 0.692 | 0.597 | 0.684 | 0.644 |
Table 9 – EPMA average chemical compositions (Wt.%) of cryptomelane-hollandite from manganese deposit Azul compared with cryptomelane-hollandite and/or hollandite from other manganese ore deposits. nd: not detected. CRY-HOL – cryptomelane-hollandite; HOL – hollandite. (1) – cryptomelane-hollandite from St Marcel-Praborna, Italia (Perseil, 1998). (2) – cryptomelane-hollandite from Manganese deposit of Vani, Milos – Greece (Liakopoulos et al., 2001). (3) – hollandite from St. Marcel-Praborna, Italy (Perseil, 1998).
| CRY-HOL | CRY-HOL | HOL | ||||
| Oxides | Ore -13A | Ore -26 | Ore 3-7B | (1) | (2) | (3) |
| SiO2 | 0.35 | 0.32 | 0.22 | 0.22 | 0.46 | 0.41 |
| Al2O3 | 5.02 | 7.31 | 1.99 | 0.00 | 0.38 | 0.00 |
| FeO | 1.45 | 0.05 | 1.63 | nd | nd | Nd |
| MnO2 | 83.32 | 84.52 | 85.12 | 83.94 | 83.52 | 75.68 |
| MgO | 0.05 | 0.01 | 0.30 | 0.00 | 0.26 | 0.00 |
| CaO | 0.08 | 0.04 | 0.56 | 0.06 | 0.45 | 0.30 |
| Na2O | 0.04 | 0.16 | 0.16 | 0.26 | 0.27 | 0.09 |
| K2O | 3.66 | 3.85 | 3.38 | 4.25 | 3.39 | 0.31 |
| TiO2 | 0.10 | 0.05 | 0.03 | 0.20 | 0.00 | 1.04 |
| P2O5 | 0.72 | 1.01 | 0.04 | nd | nd | Nd |
| SO3 | 0.06 | 0.02 | 0.04 | nd | nd | Nd |
| V2O3 | 0.42 | 0.37 | 0.09 | nd | nd | Nd |
| CoO | 0.38 | 0.29 | 0.09 | nd | nd | Nd |
| NiO | 0.06 | 0.03 | 0.09 | 0.00 | 0.10 | 0.00 |
| CuO | 0.96 | 0.64 | 0.10 | 0.00 | 0.17 | 0.00 |
| ZnO | 0.05 | 0.03 | 0.12 | 0.00 | 0.63 | 0.00 |
| As2O5 | 0.01 | 0.03 | 0.00 | nd | nd | Nd |
| SrO | 0.01 | 0.01 | 0.11 | 6.41 | 0.00 | 1.66 |
| ZrO2 | 0.02 | 0.04 | 0.00 | nd | nd | Nd |
| BaO | 1.71 | 0.98 | 0.82 | 1.52 | 6.46 | 15.91 |
| PbO | 0.09 | 0.06 | 0.02 | 0.00 | 0.30 | 0.00 |
| Total | 98.56 | 99.83 | 94.90 | 98.34 | 96.90 | 98.34 |
nd: not detected. CRY-HOL – cryptomelane-hollandite; HOL – hollandite. (1) – cryptomelane-hollandite from St Marcel-Praborna, Italia (Perseil 1998). (2) – cryptomelane-hollandite from Manganese deposit of Vani, Milos – Greece (Liakopoulos et al 2001). (3) – hollandite from St. Marcel-Praborna, Italy (Perseil 1998).
Sulphides, mainly pyrite and chalcopyrite, although small in quantity, occur in manganese-rich or not black shales (carbonaceous) and venules. They respond to the relative high concentrations of Cu, Pb, Zn, Ni, Co, Mo, As, Sb and Ag (Tables 10 and 11) together with COM. Pyrite framboids occur, associated with COM masses, sometimes with arsenopyrite and even argentite. Framboidal pyrite can be related to a series of mineralized microbial produced micro-textures (Yu et al., 2019).
Carbonaceous Organic Matter (COM) – The carbonaceous organic matter that frequently occurs in the transition from the grey to dark grey or black shales (Figures 8 B) reaches 4.7% in quantity. It is not crystalline. No graphite has been identified yet, which shows that these sedimentary rocks were not affected even by very low metamorphism, strengthened by the presence of illite (sometimes sericite) and still kaolinite.
The content of organic matter content (COM) is extremely variable (Figure 13), being more abundant, of course, in the carbonaceous shales with or without Mn-oxyhydroxides, which, together with the disseminated sulphides and the illites/sericites, give the grey colour to these rocks.
Extracts obtained by decantation in distilled water and submitted to analysis by scanning electron microscopy (SEM/EDS) and XRD confirmed the presence of amorphous carbon (Figure 14 a, b, c).
The SEM/EDS chemical analytical spectra of extracted organic matter (Figure 14 a) indicates as high amount of carbon and some Fe, Mn, K, Mg, Al, S and O, which can be related to pyrite (Fe and S), rare rhodochrosite (Mn, Mg, C, O), kaolinite (Al, Si, O) and cryptomelane (K, Mn, O) (Figure 14 b).
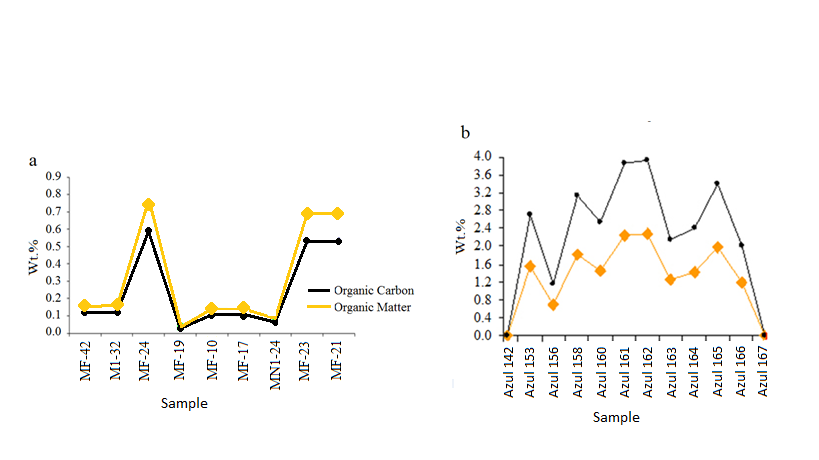
Figure 13 – (a) Contents of organic matter and organic carbon for samples coming from high grade manganese ore and (b) from carbonaceous manganese-bearing dark grey shales observed in the drill holes. Organic carbon (Corg) and Organic Matter (OM) in Wt. %.
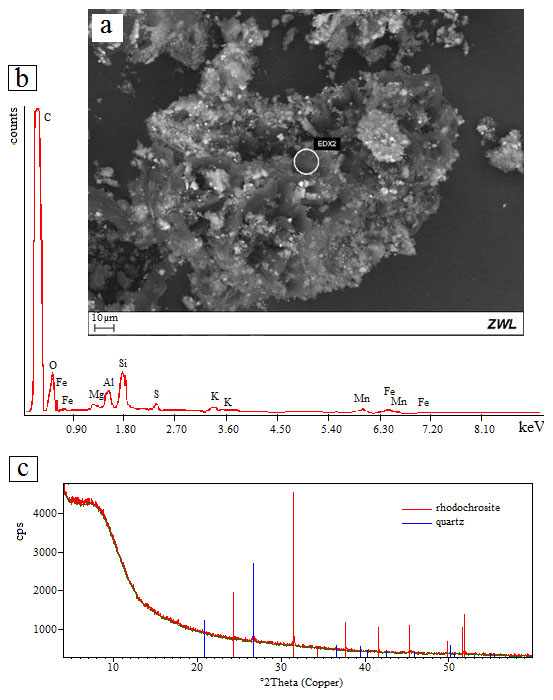
Figure 14 – (a) SEM image of extracted organic matter from dark gray manganese-rich shale; (b) EDS/SEM chemical analytical spectra of extracted organic matter (EDX2) indicating high quantity of carbon, and some of O, Si, Al, Fe, Mn, K, Mg and S, which can be related to pyrite (Fe and S), rhodochrosite (Mn, Mg, C and O), kaolinite (Al, Si and O) and cryptomelane (K, Mn and O); (c) XRD diagram for the extracted organic matter showing the domain of amorphous materials (organic matter) and some reflexes from rhodochrosite and quartz. The analyzes were carried out at ZWL, Lauf, Germany.
Whole-Rock Geochemistry – Manganese-rich dark grey shales (Table 10) are distinguished by high but variable manganese oxides (up to 80.7% MnO), followed by SiO2 (3 to 53%), Al2O3 (1.8 to 23%), Fe2O3 (1.7 to 12.7%), MgO (0.1 to 4.3), K2O (0.5 to 2.9%) and TiO2 (0.1 to 0.8%). Alternately, they are relatively poor in CaO (<1.0%), Na2O (0.2%) and P2O5 (0.2%), except for some isolated samples. The values of MnO correspond mainly to cryptomelane, while K2O corresponds to this mineral and illite/sericite, which is reinforced by the MgO contents; the SiO2 content of this mineral, quartz and still kaolinite. All Al2O3 represents aluminosilicates such as kaolinite and illite/sericite (Table 8). The highest values of CaO are referenced to the random presence of calcite and/or rhodochrosite and kutnohorite, which were randomly identified in venules and microbands in these rocks. Those more pronounced P2O5 are interpreted as possible apatites, but the mineral occurs sporadically. The TiO2 values are compatible with the rutile needles, as outlined by SEM/EDS.
Table 10 – Chemical composition (Wt.%) of manganese ore related to shale (dark grey shales). Cont´n.
| SAMPLE ID: | SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K20 | TiO2 | P205 | LOI |
| Azul 06 | 3.7 | 9.04 | 4.11 | 65.32 | 0.11 | 0.04 | 0.07 | 2,00 | 0.37 | 0.08 | 13.8 |
| Azul 09 | 5.04 | 6.39 | 4.41 | 65.94 | 0.23 | 0.19 | 0.08 | 2.35 | 0.42 | 0.44 | 13.25 |
| Azul 10 | 0.83 | 1.83 | 1.7 | 80.74 | 0.12 | 0.14 | 0.1 | 1.26 | 0.17 | 0.42 | 13.05 |
| Azul 18 | 15.29 | 13.14 | 9.97 | 41.2 | 0.72 | 0.39 | 0.09 | 1.41 | 0.56 | 0.11 | 15.68 |
| Azul 50 | 14.05 | 17.67 | 9.07 | 38.5 | 0.45 | 0.19 | 0.04 | 0.62 | 0.736 | 0.07 | 18.96 |
| Azul 81 | 35.72 | 8.06 | 5.46 | 23.36 | 1.88 | 0.52 | 0.14 | 1.9 | 0.424 | 0.06 | 22.3 |
| Azul 82 | 31.22 | 5.13 | 6.05 | 29.11 | 1.49 | 0.58 | 0.15 | 0.92 | 0.273 | 0.05 | 24.05 |
| Azul 83 | 17.42 | 2.54 | 3.46 | 45.16 | 1.48 | 1.52 | 0.18 | 0.5 | 0.112 | 0.06 | 27.92 |
| Azul 88 | 46.63 | 9.67 | 5.13 | 15.85 | 2.62 | 0.38 | 0.1 | 2.79 | 0.551 | 0.1 | 15.52 |
| Azul 89 | 23.31 | 4.44 | 2.44 | 39.46 | 1.39 | 1.18 | 0.19 | 1.06 | 0.319 | 0.06 | 24.85 |
| Azul 120 | 2.56 | 3.38 | 1.94 | 75.18 | 0.12 | 0.21 | 0.25 | 2.27 | 0.186 | 0.47 | 13.79 |
| Azul 130 | 18.15 | 19.92 | 8.18 | 35.17 | 0.36 | 0.14 | 0.03 | 0.65 | 0.808 | 0.09 | 16.46 |
| Azul 140* | 14.78 | 15.88 | 8.25 | 40.96 | 0.58 | 0.23 | 0.11 | 0.72 | 0.829 | 0.1 | 17.24 |
| Azul 142* | 6.44 | 13.15 | 5.93 | 53.28 | 0.55 | 0.22 | 0.04 | 0.93 | 0.587 | 0.1 | 17.7 |
| Azul 162 | 42.61 | 9.85 | 16.52 | 4.72 | 4.29 | 0.37 | 0.07 | 2.96 | 0.566 | 0.01 | 16.99 |
| Azul 163 | 46.72 | 9.61 | 12.7 | 6.82 | 3.85 | 0.75 | 0.33 | 2.46 | 0.464 | 0.37 | 15.02 |
| Azul 164 | 53.39 | 11.04 | 7.29 | 6.26 | 3.71 | 0.46 | 0.05 | 3.39 | 0.586 | 0.16 | 12.74 |
| Azul 165 | 39.53 | 7.77 | 9.79 | 16.85 | 3.08 | 1.33 | 0.04 | 2.47 | 0.392 | 0.16 | 18.71 |
| Azul 166 | 33.38 | 6.67 | 3.74 | 28.68 | 3.12 | 1.25 | nd | 2.38 | 0.361 | 0.17 | 20.26 |
| Azul 173* | 7.99 | 14.58 | 8.1 | 49.96 | 0.26 | 0.13 | 0.05 | 0.92 | 0.443 | 0.1 | 17.03 |
| Azul 175* | 17.17 | 16.03 | 8.87 | 41,00 | 0.2 | 0.17 | 0.19 | 1.13 | 0.741 | 0.18 | 13.53 |
| Azul 176* | 9.45 | 15.72 | 9.03 | 47.49 | 0.2 | 0.12 | 0.03 | 0.87 | 0.718 | 0.18 | 16.32 |
| Azul 178 | 24.43 | 23.32 | 12.72 | 21.84 | 0.23 | 0.13 | 0.13 | 0.76 | 0.987 | 0.19 | 14.31 |
| Azul 199 | 43.22 | 9.42 | 7.66 | 16.65 | 1.79 | 0.35 | 0.27 | 2.31 | 0.518 | 0.1 | 16.42 |
| Azul 201 | 34.33 | 7.43 | 2.33 | 31.25 | 0.88 | 0.72 | 0.14 | 1.28 | 0.283 | 0.08 | 20.35 |
| Azul 202 | 43.16 | 9,00 | 2.78 | 24.13 | 1.03 | 0.36 | 0.02 | 1.93 | 0.345 | 0.05 | 16.33 |
| SAMPLE ID. | V | Cr | Co | Ni | Cu | Zn | Ga | Ge | As | Rb | Sr | Y | Zr | Nb | Mo | Ag | In | Sn | Sb | Cs | Ba | |||||||||||||||||||||||
| Azul 06 | 160 | 24 | 526 | 122 | 503 | 226 | 27 | 1.1 | 48 | 33 | 431 | 43 | 82 | 4.2 | 27 | 1.9 | nd | 1 | 0.6 | 1.2 | 2440 | |||||||||||||||||||||||
| Azul 09 | 208 | 31 | 513 | 589 | 716 | 1790 | 20 | 0.9 | 53 | 56 | 226 | 30 | 42 | 3.3 | 131 | 1.2 | nd | nd | 2.7 | 2.8 | 2890 | |||||||||||||||||||||||
| Azul 10 | 127 | 32 | 358 | 545 | 362 | 1860 | 13 | nd | 72 | 31 | 140 | 14 | 24 | 1.9 | 133 | 0.6 | nd | nd | 1.2 | 0.5 | 409 | |||||||||||||||||||||||
| Azul 18 | 204 | 35 | 292 | 304 | 302 | 315 | 26 | 1 | 83 | 66 | 623 | 29 | 167 | 6.5 | 39 | 0.5 | nd | 2 | 3.1 | 4.2 | 2950 | |||||||||||||||||||||||
| Azul 50 | 142 | nd | 334 | 390 | 295 | 173 | 26 | nd | 25 | 51 | 150 | 41 | 194 | 7 | 12 | nd | nd | 2 | 5.1 | 5.2 | 1890 | |||||||||||||||||||||||
| Azul 81 | 168 | 90 | 112 | 212 | 254 | 761 | 13 | nd | 30 | 71 | 19 | 18 | nd | 6 | 19 | nd | nd | nd | 2.1 | 2.7 | 241 | |||||||||||||||||||||||
| Azul 82 | 130 | 88 | 162 | 325 | 225 | 551 | 11 | nd | 64 | 40 | 16 | 10 | nd | 4 | 65 | 0.6 | nd | 8 | 5.4 | 1.4 | 174 | |||||||||||||||||||||||
| Azul 83 | 135 | 35 | 64 | 340 | 84 | 673 | 10 | nd | 22 | 17 | 15 | 5 | nd | 3 | 20 | nd | nd | nd | 1.1 | 0.5 | 151 | |||||||||||||||||||||||
| Azul 88 | 174 | 105 | 70 | 128 | 293 | 492 | 16 | nd | 25 | 106 | 30 | 20 | 131 | 7 | 16 | 0.6 | nd | nd | 2.5 | 3.8 | 368 | |||||||||||||||||||||||
| Azul 89 | 173 | 46 | 57 | 337 | 50 | 439 | 12 | nd | 22 | 42 | 19 | 5 | nd | 3 | 19 | nd | nd | nd | 1.6 | 1.1 | 284 | |||||||||||||||||||||||
| Azul 120 | 127 | 25 | 309 | 470 | 554 | 1.490 | 21 | nd | 47 | 51 | 136 | 11 | nd | 1 | 34 | 0.8 | nd | nd | 1.3 | 1.3 | 2160 | |||||||||||||||||||||||
| Azul 130 | 184 | 32 | 234 | 316 | 243 | 199 | 32 | nd | 27 | 43 | 167 | 35 | 245 | 11 | 14 | nd | nd | 3 | 3.2 | 4.5 | 1900 | |||||||||||||||||||||||
| Azul 140* | 206 | 81 | 432 | 515 | 745 | 220 | 35 | nd | 27 | 64 | 180 | 45 | nd | 10 | 26 | nd | nd | 3 | 2.5 | 5.4 | 1250 | |||||||||||||||||||||||
| Azul 142* | 282 | -20 | 431 | 520 | 828 | 320 | 40 | nd | 49 | 68 | 171 | 44 | nd | 11 | 23 | 0.9 | nd | 2 | 2.9 | 5.6 | 1290 | |||||||||||||||||||||||
| Azul 162 | 165 | 96 | 62 | 131 | nd | 619 | 13 | nd | 16 | 97 | 23 | 26 | 109 | 5 | -2 | nd | nd | nd | nd | 3 | 278 | |||||||||||||||||||||||
| Azul 163 | 233 | 162 | 105 | 198 | 273 | 961 | 14 | 2 | 89 | 103 | 35 | 32 | 106 | 4 | 23 | 1.6 | nd | nd | 3.2 | 3.6 | 316 | |||||||||||||||||||||||
| Azul 164 | 226 | 119 | 76 | 116 | 105 | 793 | 17 | nd | 29 | 116 | 35 | 24 | 124 | 5 | 8 | 0.9 | nd | nd | 1 | 3.2 | 395 | |||||||||||||||||||||||
| Azul 165 | 152 | 82 | 110 | 119 | 13 | 1070 | 16 | nd | 19 | 81 | 21 | 28 | 91 | 4 | 3 | 0.6 | nd | nd | nd | 2.3 | 265 | |||||||||||||||||||||||
| Azul 166 | 207 | 74 | 103 | 166 | 195 | 943 | 19 | nd | 93 | 74 | 25 | 17 | 41 | 3 | 27 | nd | nd | nd | 1.7 | 1 | 292 | |||||||||||||||||||||||
| Azul 173* | 169 | 119 | 478 | 483 | 643 | 413 | 47 | 1 | 34 | 34 | 176 | 31 | nd | 8 | 28 | 2.3 | nd | 2 | 2.6 | 3.1 | 3560 | |||||||||||||||||||||||
| Azul 175* | 208 | 56 | 308 | 252 | 252 | 317 | 31 | nd | 28 | 33 | 130 | 33 | nd | 9 | 25 | 2.7 | nd | 2 | 4.6 | 2.8 | 1920 | |||||||||||||||||||||||
| Azul 176* | 233 | 73 | 306 | 346 | 454 | 684 | 39 | nd | 76 | 31 | 113 | 30 | nd | 10 | 53 | 2.1 | nd | 3 | 3.3 | 2.7 | 1600 | |||||||||||||||||||||||
| Azul 178 | 241 | 144 | 188 | 166 | 276 | 330 | 30 | 1 | 30 | 22 | 84 | 32 | 334 | 11 | 32 | 2.8 | nd | 3 | 3.2 | 1.3 | 3100 | |||||||||||||||||||||||
| Azul 199 | 182 | 117 | 137 | 216 | 216 | 555 | 14 | 1 | 31 | 111 | 39 | 17 | 116 | 60 | 22 | 3.2 | nd | nd | 1.3 | 3.7 | 352 | |||||||||||||||||||||||
| Azul 201 | 120 | 75 | 167 | 220 | 107 | 695 | 15 | 1 | 10 | 59 | 14 | 12 | 70 | 5 | 17 | 1.4 | nd | 1 | 1.3 | 2.7 | 167 | |||||||||||||||||||||||
| Azul 202 | 74 | 67 | 79 | 172 | 38 | 233 | 16 | 1 | 6 | 73 | 12 | 10 | 103 | 5 | 12 | nd | nd | nd | nd | 2.9 | 179 | |||||||||||||||||||||||
| SAMPLE ID. | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Tl | Pb | Bi | Th | U | ||||||||||||||||||||||
| Azul 06 | 118 | 474 | 33 | 131 | 27 | 6.6 | 17 | 2.9 | 16 | 2.7 | 6.8 | 0.9 | 5.9 | 0.7 | 3.5 | 0.4 | 2.2 | 5 | 68 | Nd | 8.7 | 4.1 | ||||||||||||||||||||||
| Azul 09 | 184 | 166 | 33 | 98 | 13 | 2.8 | 9 | 1.3 | 6.5 | 1.1 | 2.7 | 0.4 | 2.4 | 0.3 | 2.4 | 0.3 | 10 | 4.7 | 133 | Nd | 4.8 | 2.5 | ||||||||||||||||||||||
| Azul 10 | 32 | 18 | 6.9 | 23 | 3.9 | 1 | 3.3 | 0.5 | 2.6 | 0.5 | 1.2 | 0.2 | 1 | 0.1 | 0.9 | 0.1 | 3 | 1.1 | 21 | Nd | 1.5 | 1.2 | ||||||||||||||||||||||
| Azul 18 | 78 | 178 | 14 | 50 | 9.3 | 2.5 | 6.5 | 1 | 5.6 | 1.1 | 2.8 | 0.4 | 2.6 | 0.4 | 5 | 0.6 | 3.8 | 5.5 | 144 | 0.4 | 10 | 4.1 | ||||||||||||||||||||||
| Azul 50 | 47 | 150 | 14 | 56 | 14 | 3.6 | 12 | 1.9 | 10 | 1.7 | 5 | 0.8 | 4.6 | 0.6 | 5.3 | 0.7 | 3 | 2.3 | 122 | 0.7 | 10 | 2 | ||||||||||||||||||||||
| Azul 81 | 17 | 40 | 3 | 13 | 2.7 | 0.9 | 2.8 | 0.5 | 2.7 | 0.5 | 1.6 | 0.2 | 1.5 | 0.2 | 4.2 | 0.5 | 6 | 0.5 | 69 | Nd | 5 | 2.2 | ||||||||||||||||||||||
| Azul 82 | 12 | 31 | 2.2 | 9.2 | 1.9 | 0.5 | 1.8 | 0.3 | 1.8 | 0.3 | 1.1 | 0.2 | 1 | 0.2 | 2.9 | 0.3 | 6 | 0.5 | 48 | Nd | 3.7 | 1.5 | ||||||||||||||||||||||
| Azul 83 | 7.5 | 21 | 1.3 | 5.5 | 1.2 | 0.4 | 1.1 | 0.2 | 1.1 | 0.2 | 0.7 | 0.1 | 0.6 | 0.1 | 1.5 | 0.1 | 3 | 0.2 | 37 | Nd | 1.7 | 0.8 | ||||||||||||||||||||||
| Azul 88 | 21 | 49 | 3.8 | 16 | 3.4 | 1 | 3.4 | 0.6 | 3.4 | 0.6 | 2 | 0.3 | 1.8 | 0.3 | 4.3 | 0.6 | 7 | 1 | 51 | Nd | 6.3 | 2.2 | ||||||||||||||||||||||
| Azul 89 | 7.8 | 22 | 1.5 | 6.6 | 1.5 | 0.4 | 1.5 | 0.3 | 1.6 | 0.3 | 1 | 0.1 | 0.9 | 0.1 | 1.9 | 0.2 | 4 | 0.3 | 12 | Nd | 2.5 | 1.7 | ||||||||||||||||||||||
| Azul 120 | 35 | 42 | 7.4 | 34 | 7.5 | 1.9 | 7.3 | 1.2 | 6.9 | 1.2 | 2.7 | 0.3 | 2 | 0.3 | 1.4 | 0.2 | nd | 3.3 | 175 | Nd | 2.6 | 2 | ||||||||||||||||||||||
| Azul 130 | 62 | 135 | 13 | 49 | 10 | 2.3 | 7.8 | 1.3 | 7 | 1.3 | 4 | 0.6 | 3.4 | 0.5 | 8.3 | 0.9 | 6 | 2.1 | 159 | 1.2 | 14 | 2.9 | ||||||||||||||||||||||
| Azul 140* | 58 | 114 | 13 | 50 | 10 | 2.6 | 9 | 1.5 | 8 | 1.6 | 4.7 | 0.7 | 4 | 0.6 | 8.6 | 0.9 | 24 | 2.1 | 176 | 1.4 | 15 | 3.1 | ||||||||||||||||||||||
| Azul 142* | 62 | 104 | 14 | 55 | 12 | 2.9 | 10 | 1.7 | 8.9 | 1.7 | 4.9 | 0.7 | 4.1 | 0.5 | 5.9 | 0.6 | 16 | 3.9 | 283 | 1.3 | 9.8 | 2.4 | ||||||||||||||||||||||
| Azul 162 | 24 | 48 | 4.8 | 19 | 3.8 | 1 | 3.6 | 0.6 | 3.5 | 0.7 | 2.2 | 0.3 | 1.8 | 0.2 | 3.3 | 0.4 | 6 | 0.1 | 56 | Nd | 5.6 | 2.3 | ||||||||||||||||||||||
| Azul 163 | 21 | 44 | 4.5 | 19 | 4.1 | 1.1 | 4.3 | 0.7 | 4.1 | 0.8 | 2.5 | 0.3 | 1.9 | 0.3 | 2.8 | 0.4 | 6 | 0.8 | 966 | 11 | 5.5 | 2.7 | ||||||||||||||||||||||
| Azul 164 | 22 | 48 | 4.7 | 19 | 3.8 | 1 | 3.4 | 0.6 | 3.3 | 0.7 | 2 | 0.3 | 1.8 | 0.3 | 3.7 | 0.5 | 8 | 0.6 | 93 | Nd | 6.8 | 2.8 | ||||||||||||||||||||||
| Azul 165 | 20 | 41 | 4.2 | 18 | 3.8 | 0.9 | 3.7 | 0.6 | 3.6 | 0.7 | 2.1 | 0.3 | 1.7 | 0.2 | 3.1 | 0.3 | 5 | 0.2 | 44 | Nd | 5.1 | 1.9 | ||||||||||||||||||||||
| Azul 166 | 15 | 36 | 3 | 13 | 2.7 | 0.9 | 2.5 | 0.4 | 2.4 | 0.5 | 1.4 | 0.2 | 1.1 | 0.2 | 2.1 | 0.2 | 6 | 0.7 | 38 | Nd | 3.7 | 2.4 | ||||||||||||||||||||||
| Azul 173* | 65 | 247 | 18 | 68 | 16 | 3.3 | 9.9 | 1.6 | 8.5 | 1.5 | 4.2 | 0.6 | 3.7 | 0.5 | 5.1 | 0.5 | 18 | 7 | 165 | 2 | 18 | 3.4 | ||||||||||||||||||||||
| Azul 175* | 44 | 176 | 11 | 47 | 12 | 2.8 | 8.1 | 1.4 | 7.8 | 1.4 | 4.2 | 0.6 | 3.9 | 0.5 | 7.8 | 0.9 | 8 | 1.5 | 162 | 1.1 | 15 | 4.7 | ||||||||||||||||||||||
| Azul 176* | 39 | 116 | 8.8 | 34 | 7.1 | 1.8 | 5.6 | 0.9 | 5.4 | 1.1 | 3.2 | 0.5 | 2.8 | 0.4 | 6.1 | 0.8 | 26 | 3 | 216 | 2.5 | 14 | 3.4 | ||||||||||||||||||||||
| Azul 178 | 37 | 117 | 9 | 38 | 8.9 | 2.3 | 7.1 | 1.3 | 6.7 | 1.3 | 3.8 | 0.6 | 3.4 | 0.5 | 9.7 | 1.1 | 7 | 2.5 | 266 | 1.3 | 19 | 4.5 | ||||||||||||||||||||||
| Azul 199 | 21 | 51 | 4.3 | 17 | 3.2 | 0.9 | 3 | 0.5 | 2.8 | 0.6 | 1.7 | 0.3 | 1.5 | 0.2 | 3.9 | 1.3 | 4 | 0.4 | 52 | Nd | 6.8 | 17 | ||||||||||||||||||||||
| Azul 201 | 14 | 32 | 2.8 | 11 | 2 | 0.5 | 1.9 | 0.4 | 2 | 0.4 | 1.3 | 0.2 | 1.1 | 0.2 | 3.3 | 0.3 | 3 | 0.5 | 94 | Nd | 6.7 | 2.1 | ||||||||||||||||||||||
| Azul 202 | 13 | 26 | 2.2 | 8.6 | 1.7 | 0.5 | 1.5 | 0.3 | 1.6 | 0.3 | 1.1 | 0.2 | 1.1 | 0.2 | 3.8 | 0.4 | 3 | 0.6 | 12 | Nd | 5.7 | 1.6 | ||||||||||||||||||||||
Table 11 – Chemical composition of manganese-poor gray shales and siltstones, in general pyrite poor. Cont´n.
| SAMPLE ID. | SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K20 | TiO2 | P2O5 | LOI | ||||||||||||||||||||||||||||||||||||||||||
| Azul 13 | 65.12 | 16.26 | 5.93 | 0.04 | 0.80 | 0.03 | nd | 2.25 | 1.52 | 0.09 | 7.18 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 15 | 68.73 | 13.13 | 7.95 | 0.02 | 0.32 | 0.02 | 0.01 | 1.06 | 1.26 | 0.13 | 6.16 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 24 | 72.71 | 10.53 | 5.26 | 0.10 | 0.64 | 0.02 | 0.02 | 1.72 | 0.72 | 0.09 | 7.09 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 25 | 57.35 | 10.43 | 21.28 | 0.14 | 0.09 | 0.04 | 0.02 | 0.20 | 0.53 | 0.22 | 8.55 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 93 | 64.87 | 11.45 | 15.17 | 0.04 | 0.16 | 0.02 | 0.09 | 0.47 | 0.52 | 0.13 | 7.30 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 98 | 62.91 | 11.47 | 9.86 | 0.08 | 0.82 | 0.02 | 0.08 | 2.20 | 0.56 | 0.17 | 10.87 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 102 | 72.37 | 15.15 | 2.57 | 0.13 | 0.97 | nd | 0.08 | 2.35 | 0.72 | 0.04 | 6.02 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 110 | 71.96 | 13.68 | 4.42 | 0.07 | 0.36 | 0.06 | 0.22 | 1.26 | 0.57 | 0.10 | 6.26 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 114 | 63.98 | 14.22 | 5.15 | 0.31 | 2.83 | 0.14 | 0.09 | 6.23 | 0.94 | 0.02 | 6.42 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 115 | 60.31 | 13.09 | 8.44 | 0.27 | 2.25 | 0.32 | 0.09 | 3.64 | 0.79 | 0.01 | 10.44 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 153 | 68.00 | 14.11 | 3.51 | 0.08 | 1.14 | 0.02 | 0.12 | 4.30 | 0.58 | 0.04 | 6.95 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 156 | 67.20 | 14.31 | 3.09 | 0.09 | 1.48 | nd | 0.15 | 4.23 | 0.70 | 0.04 | 7.69 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 158 | 61.83 | 13.35 | 5.71 | 0.44 | 4.37 | 0.02 | 0.04 | 3.90 | 0.65 | 0.03 | 9.05 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 160 | 63.20 | 13.07 | 4.78 | 0.57 | 3.40 | 0.02 | 0.04 | 4.21 | 0.63 | 0.03 | 8.96 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 161 | 54.80 | 11.86 | 10.27 | 1.30 | 3.29 | 0.05 | 0.06 | 3.67 | 0.55 | nd | 12.97 | ||||||||||||||||||||||||||||||||||||||||||
| Azul 84 | 65.59 | 14.28 | 4.09 | 0.32 | 3.89 | 0.02 | 0.04 | 1.19 | 0.89 | 0.04 | 8.82 | ||||||||||||||||||||||||||||||||||||||||||
| SAMPLE ID. | V | Cr | Co | Ni | Cu | Zn | Ga | Ge | As | Rb | Sr | Y | |||||||||||||||||||||||||||||||||||||||||
| Azul 13 | 228.98 | 101.97 | 1.19 | 20.68 | 21.73 | -30.00 | 20.60 | 1.99 | 21.13 | 91.38 | 25.25 | 68.91 | |||||||||||||||||||||||||||||||||||||||||
| Azul 15 | 159.29 | 92.44 | nd | Nd | 33.28 | 37.43 | 17.73 | 1.79 | 25.38 | 45.33 | 32.36 | 53.37 | |||||||||||||||||||||||||||||||||||||||||
| Azul 24 | 228.68 | 125.81 | 2.51 | 24.42 | 141.31 | -30.00 | 16.43 | 2.69 | 126.55 | 67.45 | 33.05 | 26.58 | |||||||||||||||||||||||||||||||||||||||||
| Azul 25 | 290.38 | 104.90 | 2.28 | 22.28 | 269.48 | -30.00 | 11.90 | 1.97 | 9.42 | 9.09 | 39.00 | 23.61 | |||||||||||||||||||||||||||||||||||||||||
| Azul 93 | 102.25 | 102.01 | 4.73 | 41.02 | 34.70 | 92.51 | 17.18 | 2.21 | 13.92 | 27.17 | 10.52 | 17.55 | |||||||||||||||||||||||||||||||||||||||||
| Azul 98 | 264.13 | 108.46 | 12.58 | 40.52 | 317.31 | 50.24 | 13.48 | 1.55 | 70.83 | 93.47 | 30.58 | 31.03 | |||||||||||||||||||||||||||||||||||||||||
| Azul 102 | 93.92 | 139.20 | 6.45 | 43.34 | 46.68 | 55.57 | 19.30 | 1.29 | nd | 111.26 | 6.88 | 13.07 | |||||||||||||||||||||||||||||||||||||||||
| Azul 110 | 67.60 | 95.14 | nd | Nd | 85.45 | nd | 20.60 | 1.71 | nd | 49.61 | 17.27 | 65.94 | |||||||||||||||||||||||||||||||||||||||||
| Azul 114 | 115.26 | 169.48 | 17.40 | 35.19 | 104.32 | 144.09 | 20.79 | 1.67 | 10.66 | 157.30 | 46.12 | 9.63 | |||||||||||||||||||||||||||||||||||||||||
| Azul 115 | 107.87 | 173.44 | 27.82 | 112.57 | 90.17 | 222.41 | 20.58 | nd | nd | 111.99 | 26.76 | 9.75 | |||||||||||||||||||||||||||||||||||||||||
| Azul 153 | 100.00 | 113.00 | 9.00 | 44.00 | 118.00 | 117.00 | 16.00 | 2.00 | 10.00 | 134.00 | 43.00 | 24.00 | |||||||||||||||||||||||||||||||||||||||||
| Azul 156 | 143.00 | 125.00 | 7.00 | 46.00 | 86.00 | 147.00 | 17.00 | 2.00 | 9.00 | 153.00 | 37.00 | 21.00 | |||||||||||||||||||||||||||||||||||||||||
| Azul 158 | 149.00 | 114.00 | 31.00 | 81.00 | 189.00 | 526.00 | 16.00 | nd | 15.00 | 144.00 | 29.00 | 26.00 | |||||||||||||||||||||||||||||||||||||||||
| Azul 160 | 242.00 | 121.00 | 32.00 | 108.00 | 465.00 | 386.00 | 16.00 | nd | 36.00 | 154.00 | 33.00 | 24.00 | |||||||||||||||||||||||||||||||||||||||||
| Azul 161 | 148.00 | 114.00 | 35.00 | 115.00 | 12.00 | 382.00 | 14.00 | nd | 156.00 | 123.00 | 35.00 | 31.00 | |||||||||||||||||||||||||||||||||||||||||
| Azul 84 | 110.26 | 185.62 | 33.32 | 109.71 | 91.25 | 106.36 | 18.02 | 2.03 | nd | 55.58 | 12.62 | 15.83 | |||||||||||||||||||||||||||||||||||||||||
| SAMPLE ID. | Zr | Nb | Mo | Ag | In | Sn | Sb | Cs | Ba | Hf | Ta | W | Tl | Pb | Bi | ||||||||||||||||||||||||||||||||||||||
| Azul 13 | 215.16 | 9.27 | 10.35 | 3.53 | 0.13 | 1.93 | 0.98 | 3.27 | 447.28 | 5.85 | 0.74 | 1.94 | 0.19 | 9.75 | nd | ||||||||||||||||||||||||||||||||||||||
| Azul 15 | 262.56 | 9.51 | 4.94 | 2.27 | Nd | 1.18 | 1.39 | 1.72 | 666.95 | 7.04 | 0.75 | 3.02 | 0.22 | 26.54 | nd | ||||||||||||||||||||||||||||||||||||||
| Azul 24 | 154.04 | 7.75 | 24.09 | 7.94 | Nd | 1.35 | 5.51 | 2.69 | 233.30 | 4.60 | 0.60 | 7.80 | 0.20 | 22.69 | nd | ||||||||||||||||||||||||||||||||||||||
| Azul 25 | 86.14 | 5.59 | 23.66 | 5.25 | 0.21 | Nd | 1.45 | 0.53 | 241.80 | 2.67 | 0.35 | 12.69 | 0.07 | 72.71 | nd | ||||||||||||||||||||||||||||||||||||||
| Azul 93 | 191.38 | 8.56 | 2.66 | Nd | Nd | 1.13 | 1.01 | 1.02 | 349.30 | 5.16 | 0.83 | 2.05 | 0.12 | 24.90 | nd | ||||||||||||||||||||||||||||||||||||||
| Azul 98 | 143.28 | 7.31 | 38.35 | 0.90 | Nd | Nd | 3.40 | 4.27 | 244.97 | 4.55 | 0.63 | 2.73 | 0.73 | 110.66 | nd | ||||||||||||||||||||||||||||||||||||||
| Azul 102 | 234.81 | 9.28 | 7.31 | Nd | Nd | 1.74 | Nd | 5.72 | 89.50 | 6.71 | 1.02 | 5.74 | 0.51 | 24.92 | nd | ||||||||||||||||||||||||||||||||||||||
| Azul 110 | 235.27 | 9.60 | nd | Nd | Nd | Nd | 1.26 | 1.73 | 189.45 | 6.48 | 0.75 | 2.07 | 0.25 | 14.76 | nd | ||||||||||||||||||||||||||||||||||||||
| Azul 114 | 219.41 | 8.45 | 2.60 | Nd | Nd | 1.65 | 1.10 | 6.20 | 603.91 | 6.35 | 0.99 | 2.50 | 1.67 | 34.75 | nd | ||||||||||||||||||||||||||||||||||||||
| Azul 115 | 180.97 | 6.83 | nd | Nd | Nd | 1.31 | 1.27 | 5.56 | 512.91 | 5.20 | 0.67 | 2.44 | 1.27 | 25.48 | 0.43 | ||||||||||||||||||||||||||||||||||||||
| Azul 153 | 176.00 | 6.00 | 20.00 | Nd | Nd | 1.00 | 0.70 | 4.00 | 578.00 | 4.70 | 0.50 | 3.00 | 1.10 | 59.00 | nd | ||||||||||||||||||||||||||||||||||||||
| Azul 156 | 188.00 | 6.00 | 15.00 | Nd | Nd | 1.00 | 1.00 | 5.00 | 620.00 | 5.10 | 0.60 | 3.00 | 0.70 | 139.00 | 2.10 | ||||||||||||||||||||||||||||||||||||||
| Azul 158 | 156.00 | 7.00 | 4.00 | 0.70 | Nd | 1.00 | 0.90 | 5.40 | 467.00 | 4.60 | 0.60 | 5.00 | 1.60 | 50.00 | nd | ||||||||||||||||||||||||||||||||||||||
| Azul 160 | 169.00 | 7.00 | 18.00 | 0.70 | Nd | 1.00 | 4.20 | 4.90 | 475.00 | 4.90 | 0.60 | 6.00 | 1.60 | 63.00 | nd | ||||||||||||||||||||||||||||||||||||||
| Azul 161 | 137.00 | 7.00 | 2.00 | 0.70 | Nd | Nd | 0.60 | 3.70 | 428.00 | 4.00 | 0.50 | 5.00 | 0.20 | 40.00 | nd | ||||||||||||||||||||||||||||||||||||||
| Azul 84 | 211.14 | 9.02 | nd | Nd | Nd | 1.28 | 1.65 | 2.71 | 253.70 | 5.96 | 0.85 | 5.44 | 0.42 | 22.65 | nd | ||||||||||||||||||||||||||||||||||||||
| SAMPLE ID. | Th | U | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||||||||||||||||||||||||||||||||||
| Azul 13 | 9.77 | 3.61 | 17.57 | 28.87 | 4.08 | 21.37 | 5.63 | 1.66 | 6.64 | 1.22 | 7.29 | 1.67 | 4.96 | 0.77 | 4.52 | 0.64 | |||||||||||||||||||||||||||||||||||||
| Azul 15 | 11.03 | 3.49 | 22.47 | 36.05 | 5.15 | 24.40 | 4.99 | 1.47 | 5.52 | 1.01 | 6.03 | 1.36 | 4.02 | 0.64 | 3.62 | 0.52 | |||||||||||||||||||||||||||||||||||||
| Azul 24 | 8.67 | 3.25 | 33.89 | 60.90 | 6.90 | 25.90 | 4.93 | 1.40 | 4.53 | 0.73 | 3.80 | 0.80 | 2.13 | 0.32 | 1.89 | 0.28 | |||||||||||||||||||||||||||||||||||||
| Azul 25 | 4.62 | 4.34 | 23.74 | 51.31 | 4.23 | 15.69 | 3.01 | 1.16 | 2.75 | 0.51 | 3.02 | 0.70 | 1.94 | 0.29 | 1.72 | 0.25 | |||||||||||||||||||||||||||||||||||||
| Azul 93 | 11.33 | 3.00 | 13.22 | 19.98 | 2.17 | 9.66 | 2.57 | 0.74 | 2.97 | 0.52 | 3.00 | 0.63 | 1.98 | 0.30 | 1.92 | 0.30 | |||||||||||||||||||||||||||||||||||||
| Azul 98 | 7.99 | 3.00 | 31.57 | 64.28 | 6.38 | 29.57 | 6.44 | 2.01 | 6.70 | 1.05 | 5.23 | 0.95 | 2.63 | 0.33 | 2.02 | 0.28 | |||||||||||||||||||||||||||||||||||||
| Azul 102 | 9.69 | 2.28 | 31.97 | 68.76 | 5.65 | 22.77 | 3.96 | 0.92 | 2.89 | 0.44 | 2.40 | 0.47 | 1.61 | 0.24 | 1.58 | 0.23 | |||||||||||||||||||||||||||||||||||||
| Azul 110 | 13.25 | 6.76 | 26.62 | 47.30 | 4.72 | 20.71 | 4.82 | 1.41 | 6.45 | 1.43 | 9.79 | 2.15 | 6.39 | 1.00 | 6.21 | 0.92 | |||||||||||||||||||||||||||||||||||||
| Azul 114 | 8.47 | 3.09 | 11.39 | 22.91 | 2.22 | 10.02 | 2.15 | 0.67 | 2.12 | 0.36 | 2.02 | 0.39 | 1.28 | 0.20 | 1.37 | 0.21 | |||||||||||||||||||||||||||||||||||||
| Azul 115 | 6.94 | 2.75 | 14.93 | 31.05 | 2.90 | 12.74 | 2.61 | 0.67 | 2.21 | 0.36 | 1.93 | 0.37 | 1.18 | 0.18 | 1.13 | 0.18 | |||||||||||||||||||||||||||||||||||||
| Azul 153 | 9.30 | 3.30 | 32.60 | 57.90 | 7.12 | 27.30 | 5.10 | 1.28 | 4.80 | 0.70 | 3.90 | 0.80 | 2.30 | 0.32 | 1.80 | 0.29 | |||||||||||||||||||||||||||||||||||||
| Azul 156 | 8.50 | 3.00 | 25.00 | 41.70 | 4.63 | 18.20 | 3.50 | 0.95 | 3.30 | 0.50 | 2.90 | 0.60 | 1.90 | 0.29 | 1.70 | 0.26 | |||||||||||||||||||||||||||||||||||||
| Azul 158 | 7.70 | 2.70 | 24.80 | 51.90 | 5.51 | 22.50 | 4.30 | 1.11 | 3.70 | 0.60 | 3.60 | 0.70 | 2.20 | 0.32 | 2.00 | 0.26 | |||||||||||||||||||||||||||||||||||||
| Azul 160 | 8.20 | 3.30 | 26.20 | 51.00 | 5.32 | 22.00 | 4.40 | 1.18 | 4.10 | 0.70 | 3.50 | 0.70 | 2.10 | 0.31 | 1.90 | 0.27 | |||||||||||||||||||||||||||||||||||||
| Azul 161 | 6.90 | 2.60 | 22.70 | 45.20 | 4.72 | 19.20 | 4.10 | 1.04 | 4.40 | 0.80 | 4.40 | 0.90 | 2.60 | 0.36 | 1.90 | 0.27 | |||||||||||||||||||||||||||||||||||||
| Azul 84 | 8.78 | 3.07 | 20.37 | 41.69 | 3.91 | 16.88 | 3.71 | 0.95 | 3.45 | 0.54 | 3.06 | 0.57 | 1.83 | 0.27 | 1.61 | 0.24 | |||||||||||||||||||||||||||||||||||||
The content of the trace elements (Table 10), when compared to the shales generally and Mn-poor black shales from Azul specifically (Table 11) and to the crustal average, the manganese-rich dark grey shales of Azul are enriched in Ni, Cu, Zn, As, Mo, Ag and Sb and they reflect the dominance of carbonaceous organic matter with sulphides. The contents of Ni, Co and Cu also show a significant positive correlation to Mn contents, in this case to Mn-oxyhydroxides. These manganese-rich rocks/ores are also relatively enriched in Ba, Pb, and Sr and even REE, showing an additional slightly good positive correlation to MnO. Of these elements, Ba is by far the most abundant, and forms the cryptomelane-hollandite series. Among the REE, the samples that present rhodochrosite with much more frequency are poorer in these elements, being below the Earth crust and PAAS levels, while in the samples dominated by cryptomelane and cryptomelane-hollandite, REE, especially cerium, are slightly enriched.
Manganese-poor grey shales, composed of illite-sericite, quartz, chlorite and carbonaceous organic matter, usually contain pyrite and are then dominated by SiO2, Al2O3, Fe2O3, MgO (0.3 to 4.4%) and K2O (0.2 to 6.2%), in addition to TiO2 (0.5 to 1.5%) (Table 10). In turn they are comparatively poor in MnO, CaO, Na2O and P2O5 (0.01 to 0.22).
In terms of trace elements, the dark grey shales are distinguished from manganese-rich dark or black shales mainly by their lower contents of Co, Ni, Cu, Zn, As, Mo, Ag and Sb, and Ba, Sr, Pb and REE. This is explained by the absence of Mn-oxyhydroxides, mainly cryptomelane-hollandite, which are carrier of Cu, Co and Ni, as well as Ba, Sr, Pb and REE. Alternately, these Mn-poor shales display much more pyrite and organic matter, that carries (As, Mo, Sb, Ag).
DISCUSSIONS
Geological Records – The Azul manganese oxyhydroxides lenses hosted by carbonaceous black shales, siltstones/mudstones and red fine sandstones, can be well correlated to well-known Paleoproterozoic to Neoproterozoic manganese deposits worldwide, especially those of 2.4 to 2.0 Ga, such as Kalahari in Africa (Roy, 2006; Kuleshov, 2017 Dubois et al., 2017) and the Upper Proterozoic marine sequence of the Penganga Group, Andhra Pradesh, India (Roy et al., 1990). They are associated with black shales, deposited in the platform shelf by transgression episodes, at the time of the increase of the oxygen in the terrestrial atmosphere and burial of the organic matter in increasing production. These conditions can be compared to those described by Frakes and Bolton (1992), combining specific ocean chemistry, sea level and climate on the formation of primary sedimentary manganese deposits. The age obtained here, approximately 2.1 Ga, is in accordance with the data reported by Mougeot et al (1996) and Fabre et al. (2011) and discussed by Cabral et al (2013, 2017), considering mainly the black shales associated with Carajás BIF Formation. The siltstones and red sandstones represent the partial terrestrial contribution and reflect the availability of oxygen for Fe and Mn oxidation, and for organic matter hosted in the dark to black shales (Roy, 2006; Dubois et al., 2017).
Even in the Mesoarchean deposits, in which the sedimentary sequence has already been modified by metamorphism, tectonic deformations and hydrothermal activities, this picture is still possible to observe (Ghosh et al. 2015). The mode of geological occurrence of these layers in Azul is fully in agreement with the dominant general picture in the Early Proterozoic in all general aspects, including the accumulation of organic matter and the marked presence of black shales and Mn oxides. Some deposits in turn are dominated by Mn carbonates (rhodochrosite, manganoan dolomites, kutnahorite and Mn-calcite), such as the Paleoproterozoic deposits from Franceville in Gabon (Dubois et al., 2017) and Kalahari (Gutzmer & Beukes, 1996; Roy, 2006; Gutzmer et al., 2012); the Neoproterozoic deposits from Datangpo formation, South China (Yu et al., 2016, 2019), and Molango in Mexico (Roy, 2006); Mesozoic deposits from Úrkút in Hungary (Polgári et al., 2016); and Cenozoic deposits from Nikopol (Ukraine) and Chiatura (Georgia) (Roy, 2006). In all of these deposits over a wide geological time, rhodochrosite and the other carbonate minerals are of diagenetic formation, always related to oxidation of organic matter and reduction of previous amorphous manganese oxides (Tang & Liu, 1999; Roy, 2006; Gutzmer et al., 2012, Polgári et al, 2016; Yu et al., 2019).
In the Azul manganese deposits, rhodochrosite, however, is rare. This statement contrasts with what was presented by Beauvais (1984) and Beauvais et al. (1987) after Bernardelli and Beisiegel (1978), rhodochrosite being the primary source for lateritic supergene manganese ore from the Azul deposit. Two manganese-bearing units, rhodochrosite being the main mineral, were represented by carbonaceous manganese-bearing limestones or marls (Bernardelli & Beisiegel, 1978; cited by Beauvais et al., 1987). These two units were only found in a single drill hole (DH 5, Figure 2) carried out in the early 1970s. In the countless deep drill holes made later, these two rhodochrosite-bearing units were not identified. However, rhodochrosite has been identified as a sporadic mineral in isolated samples of black shales and in venules, but never as a main rock mineral in Azul deposits in the last four decades. The extreme variations in the chemical composition of rhodochrosites or even manganoan dolomites are common in the most diverse manganese deposits and are always linked to the diagenetic formation in the sedimentary package from Paleoproterozoic through Cenozoic (Fan & Yang, 1999; Varentsov, 2002; Roy, 2006; Polgári et al., 2016; Yu et al., 2016; Dubois et al., 2017; Kuleshov, 2017; Rajabzadeh et al., 2017).
Economic sedimentary deposits of manganese oxyhydroxides mainly as cryptomelane are also known in the Neoproterozoic, with strong evidence of mediation by microorganisms, such as Urucum, in Mato Grosso, Brazil (Biondi et al., 2020),
Several of these Early Proterozoic deposits are metamorphosed (India and Brazil; in Brazil: Conselheiro Lafaiete, Buritirama, Sereno, Serra do Navio and Jequié) in general Archaean (≥ 2.6 Ga) (Roy, 2006; Kuleshov, 2017; Araújo & Sousa, 2018; Salgado et al., 2019). The presence of illite, kaolinite and amorphous organic matter clearly shows that the Azul manganese deposits, and its hosting rocks were not metamorphosed, although the sedimentary packets show deformation structures. The studies of Fabre et al (2011) came to the same conclusions, with maximal to anchimetamorphic conditions indicated by sericite formation. Some 40K/39Ar ages performed on red hosting siltstones (Table 3) show ages compatible with deposits in the range of 2.3 to 2.0 Ga., in the range of single age of 1.8 Ga presented by Gomes et al (1975), Bonhomme et al (1982), Mougeot et al (1996) and Fabre et al. (2011).
The oldest folding consists of tight folds with E-W axis (Pinheiro & Holdsworth, 2000), observed in the Mine Carrapato and Mine 1 (Figures 5 and 6) and is compatible with the Trans-Amazonian (Transamazônica) tectonic deformation vector (2.1 to 1.8 Ga) (Cabral et al., 2017). The strong degree of deformation, paradoxically incompatible with the absence of metamorphism or anchimetamorphism only, can be interpreted as an active deformation that occurred when the sediments were still unconsolidated, providing a predominantly plastic deformation, which is consistent with the age of the Águas Claras sediments. This is indicated by the common presence of convolute structures observed in the mudstones/siltstones.
The next folding event is formed by smooth folds of the N-S axis, with a long wavelength, which forms sequences of synforms and antiforms, exemplified by the large antiform of the western portion of Mine 1 (Figures 2 and 5). This deformation is compatible with the Brazilian (Brasiliana) tectonics recognized by Pinheiro & Holdsworth (2000).
The main fault zone in the region is the Carajás Fault installed at 2.7 Ga, which was reactivated at 1.8 Ga, affecting the manganese mineralization by remobilization Mn-oxyhydroxides (pyrolusite, nsutite, manganite) followed by an intense silicification and kaolinization of the sediments established along faults and apical fold zones.
Mineralogical Records – In addition to the predominant cryptomelane and/or cryptomelane-hollandite, other Mn-oxyhydroxides, such as birnessite, todorokite, lithiophorite, nsutite, pyrolusite, manganite, etc., constitute the ore manganese minerals in the shales and their fault-deformed bodies. This mineralogical composition, although varying in the proportions of each species, and in the presence or absence of some of them, is typical of most of the world’s deposits of sedimentary manganese, from Proterozoic to Cenozoic (Roy, 2006; Polgári et al., 2012; Johnson et al., 2016: Biondi and Lopez; Kuleshov, 2017; Molnár et al., 2017).
In the Azul deposits, cryptomelane and cryptomelane-hollandite (Table 2) are the most abundant manganese ore mineral, however in the world deposits according to Kuleshov (2017) they lose to rhodochrosite and braunite. Braunite was not identified in Azul, and rhodochrosite is very restricted, although mentioned as protore for supergene ore formation in first publications (Bernardelli & Beisiegel, 1978 and Beauvais et al., 1987) Although rhodochrosite may constitute an important mineral ore, it is also the Mn-proto mineral for supergene enrichment, which is dominated by manganese oxyhydroxides in Africa, China and Brazil (Maynard, 2004; Chisonga et al., 2012; Putter et al., 2015; Deng et al., 2016; Kuleshov, 2017; Scarpelli & Horikava, 2017). However, cryptomelane would be the most abundant when we consider the deposits formed only by Mn oxyhydroxides. Cryptomelane and braunite are also the main manganese ores in the large Neoproterozoic Urucum deposit, which is diagenetic in origin (Biondi and Lopez, 2017); of the Neoproterozoic Mn-oxyhydroxides, although without reporting on which minerals, of Nanhua basin Mn deposits, South China, which are related to hydrothermal activity and glaciation (Yu et al., 2016) and even in Miocene Mn-oxide hydrothermal mineralizations of Boléo, Mexico (Conly et al, 2011).
As exposed, the minerals of the cryptomelane group, i.e. ryptomelane (K) hollandite (Ba), strontiumelane (Sr) and coronadite (Pb), especially the first two minerals, have been reported in several manganese deposits from Proterozoic to Cenozoic, being of sedimentary (early diagenesis), metamorphic, hydrothermal and supergene origin. For Johnson et al (2016), the manganese mineral formation begins with the deposition of Mn(IV) oxides, leading to the early diagenesis production of carbonate phases, such as rhodochrosite and so on. At Azul, Mn oxides alone predominate, that is, the conditions for Mn(IV) reduction and OM oxidation did not prevail.
In the Azul deposits, EPMA analyses show the chemical variations characteristic of the main members of the group in which the trace elements (Co, Ni, Cu and Zn) tend to concentrate in the hollandite member (Figure 15 for Zn, Ni and Cu), Cu in cryptomelane. Based on the K2O and BaO values obtained by EPMA, it was possible to individualize three chemically distinct cryptomelane-hollandites (Figure 16): Ba-free cryptomelane (group 1) representing those typically related to amorphous Mn-oxyhydroxides and sedimentary in nature; K2O/BaO = 3.0 cryptomelane-hollandites (group 2), related to deformation structures and epigenetic in nature (Table 2); and K2O/BaO variable cryptomelane-hollandites, which may be related to early diagenetic recrystallization of amorphous Mn-oxyhydroxides (Table 2).
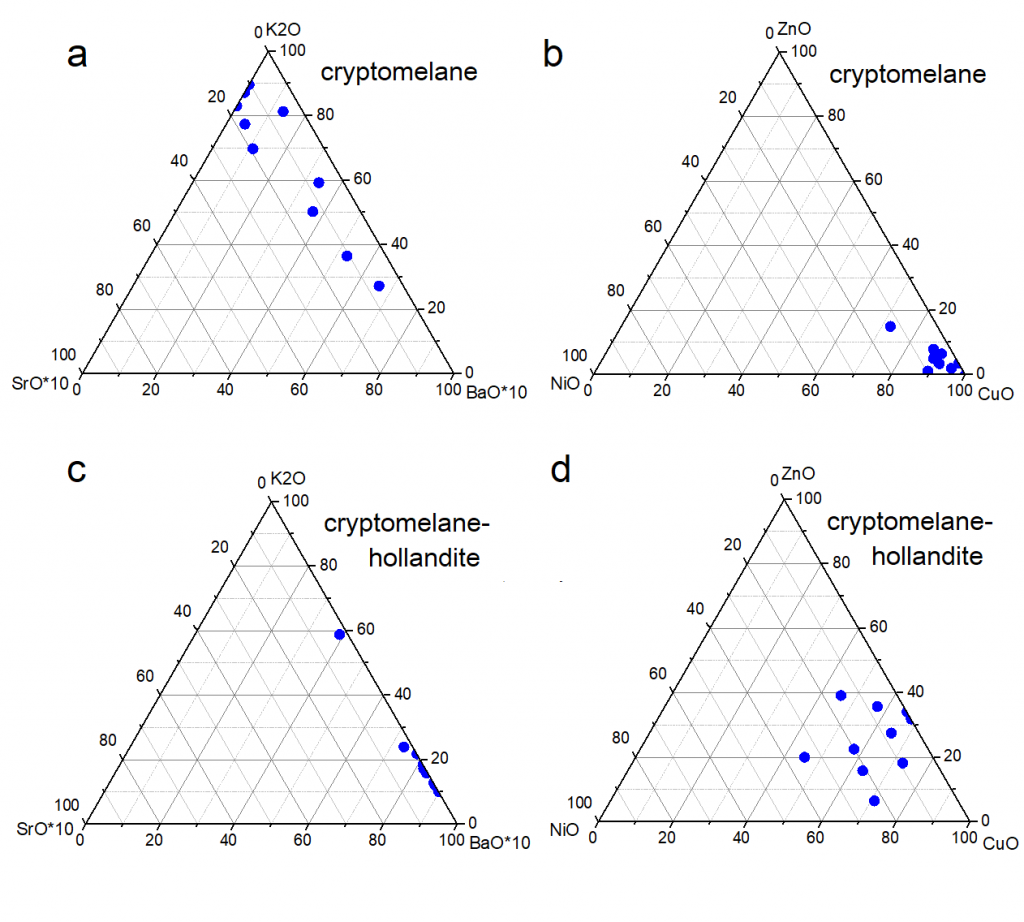
Figure 15 – Chemical composition diagrams (K2O-SrO-BaO and ZnO-NiO-CuO) for the domain of crytptomelane (a, b) and cryptomelane-hollandite (c, d) from manganese ore deposit of Azul.
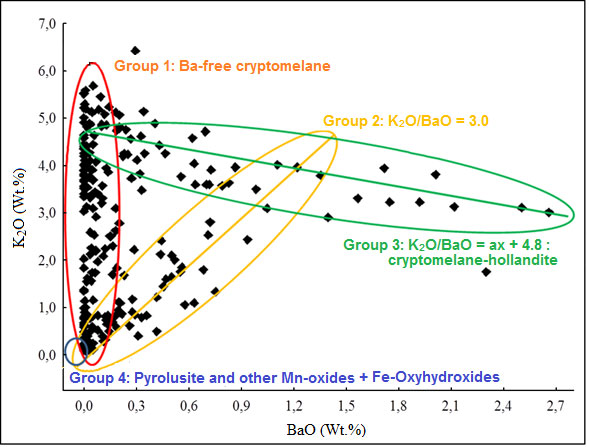
Figure 16 – K2O – BaO diagram showing the variable chemical composition of mineral series cryptomelane-hollandite Groups 2 and 3) and cryptomelane member (Group 1, Ba-free cryptomelane), and other Mn-oxides (Group 4).
Costa et al. (2005) and Araújo & Sousa (2018) found that cryptomelane and cryptomelane-hollandite also constituted the main minerals of supergene deposits of lateritic origin (Table 2), together with todorokite, lithiophorite, pyrolusite, nsutite and Mn-free minerals such as gibbsite, kaolinite, goethite, and haematite. For rhodochrosite, even as a rare mineral in the Azul mine, its diagenetic origin should be similar to those found in most manganese deposits associated with Mn-carbonate-rich shales, marl and limestones from Proterozoic through Cenozoic. The extreme variation of the chemical composition of Azul rhodochrosite indicates also its diagenetic origin as observed by Kuleshov (2017) in other deposits in a limited anoxic environment (Du et al., 2011). The diagenetic activities in Azul were more restricted, certainly as a consequence of the high oxygen availability at their precipitation time. The large set of red siltstones to sandstones that host the mineralization, confirm the oxygen availability, consuming much of it and protecting the in-built lenses of carbonaceous shales.
The constant presence of pyrite, dispersed in the shales and manganese-rich black shales, support a possible, however restricted, microbial marine sulphate reduction during the early diagenesis reactions, preserving a good portion of the carbonaceous organic matter. Pyrite and kaolinite veinlets, in turn, indicate the tectonic remobilization and epigenetic formation (Table 2).
Microbial Activity and Stromatolite-Like Structures – Stromatolites are organic sedimentary structures produced by sediment trapping, binding and/or precipitation as a result of the growth and metabolic activity of microoorganisms (Perry et al., 2007). The ubiquity of carbonaceous organic matter (COM) associated with manganese mineralizations is undeniable and has been linked to it since the Archean, represented by bacteria, algae, and protozoa (Roy, 2006; Maynard, 2004; Kuleshov, 2017; among many others), compatible with its relationship with the black shales. The abiotic oxidation of Mn(II) is kinetically inhibited below a pH of 9, and therefore Mn(II) oxidation in circumneutral natural aquatic environments is predominantly driven by biological processes (Zeiner et al., 2006). Stromatolite-like structures and microbialites have also been reported in several ancient manganese deposits, still recorded in the Archean and extending to the Proterozoic in Chitradurga Group (India), Gandarela Formation (Brazil) and South Africa (Roy, 2006; Kuleshov, 2017); at some extension in the Neoproterozoic deposit at Urucum, in Mato Grosso, Brazil (Biondi And Lopez, 2017 and 2020) and Datangpo Formation manganese deposits, South China (Yu et al., 2019); in the Mesozoic deposits of Úrkút (Polgári et al., 2012, 2016; Molnár et al., 2017); and Abadeh-Tashk area in Iran (Rajabzadeh et al., 2017) and even in recent deposits in deep-sea nodules (Akai et al., 2013). COM are a fundamental feature of sedimentary manganese formations throughout the Earth’s evolution (Maynard, 2004; Roy, 2006; Kuleshov, 2017). Economic sedimentary manganese oxyhydroxide deposits of Urucum show evidence of microbial mediation by microorganisms, especially the layers of manganese ore Mn-2 and Mn-3 with kremydillite (Biondi et al., 2020) and stromatolite-like structure just below the Mn-1 layer (Biondi and Lopez, 2017). Even in the youngest deposits of the Jurassic-Cretaceous, syngenetic and diagenetic manganese oxyhydroxides have formed, almost mediated by microorganisms, as well as in recent deep-sea nodule deposits (Akai et al., 2013). Microbial mediated reactions between manganese oxide and organic matter promote the formation of the manganese carbonate in several manganese deposits from Paleoproterozoic through Cenozoic (Gutzmer and Beukers, 1996: Fan et al., 1999; Polgári et al., 2012; Dubois et al., 2017; Yu et al., 2019). Stromatolite-like macroscopic features with Fe-rich biomats as Fe-microbialites have been described in Iranian manganese deposits (Rajabzadeh et al, 2017). Additionally, to Mn-oxyhydroxides occur silicates and carbonates, being the oxyhydroxides in part amorphous (Rajabzadeh et al, 2017), similarly to the world-class Jurassic manganese Úrkút deposit, in Hungary, with cherty Fe-Mn oxide ore being related to stromatolite-like structures. The bulk mineralogy of this ore comprises ferrihydrite, goethite, hollandite, pyrolusite, cryptomelane, todorokite, or birnessite, hausmannite and manganite, (Polgári et al, 2012; Molnár et al., 2017), even in the manganese black shales with predominate rhodochrosite microbialites (Polgári et al., 2016).
Recently, manganese rock material with stromatolite-like structures was found in mine 2 (Figures 5 and 17) of the Azul manganese deposits. Two samples were collected, ESTMn01 and MUGEO 2460. The large and most representative sample MUGEO 2460 was deposited at Museu de Geociências (Geosciences Museum) of Federal University of Pará, in Brazil. This sample (Figure 17) is dark grey in colour, very dense, and dominated by Mn-oxyhydroxides. Externally, it consists of several mat-mounds (Figure 17 A) (see Polgári et al., 2012 p. 96 Figure 7i and p. 98 Figure 8 e-h for comparison) formed by plane-parallel fine layers (Figure 17 A and B). They are sectioned by kaolin and quartz venules, sometimes with pyrite. Internally, the mounds converge first to a mass that is slightly stratified and then to a mass formed by centimetre large nodules of the same material (Figure 17 C and D), which describe layers, in part cracked and cross-cut by massive Mn-oxides (with metallic lustre and grey in colour) and Fe-hydroxides (brownish) (Figure 17 C and D). Microdiapires and microcrystalline quartz or quartz fine sand injections can also be observed (Figure 17 C and D). As a whole, part of this sample can be correlated to that described by Molnár et al. (2017, p. 844). Cryptomelane and todorokite are the main mineral of the sample MUGEO 2460, being cryptomelane-hollandite in the sample ESTMn01. Cryptomelane is a unique mineral of the massive grey crack’s infillings (Figure 17 C) displayed in MUGEO 2460 (Figure 17 C), while quartz, kaolinite and goethite are the other minerals of both samples, which generally constitute a thin capping of the entire structure (Figure 17 A).

Figure 17 – (A) A large sample of cryptomelane stromatolite-like structure formed by several mounds and/or mats (Carrapato, Azul mine, Figures 5 and 6) crosscut by kaolin veinlets sometimes concordant to fine beddings. The sample is 17 cm large. (B) Details of some mounds or mats fine stratified. (C) A cross section through the same sample showing fine parallel lamination, kaolin veins and pockets (white), massive manganese veinlets and cracks (gray) and uneven iron hydroxide veinlets (in brown). (D) Details of the previous cross section. From this section microsamples were collected for SEM/EDS analyzes showing in the Figures 18 and 19 and Table 13.
The chemical composition of two Mn-rich stromatolite-like samples (Table 12) shows content similarities for (Ca, Mg, Na, Mn, P, LOI, C, S, Cr, Cs, Ga, Rb, Sn, Y, As, Bi, In, Sb, Co, Li, Zn, La, Gd, Tb, Tm, Yb, Lu); however, the sample ESTMn01 shows higher values for Al, Fe, K, Ti, Cr, Ba, Sr, Rb, Zr, Nb, Ta, Hf, Sc, Th, U, Hg, Tl, Ce, La, Nd, Pr and Sm. Aluminium reflects the presence of kaolinite and K, Ba, Sr, Rb, Tl and LREE (Ce through Sm) + Sc of cryptomelane-hollandite, the main mineral of the samples; Zr and Hf, Nb, Ta, Th and U exhibit some isolated zircon grains and Fe, Cr and Hg the goethite. What draws much attention in these two samples is the high levels of SiO2 and MnO, which together and alone make up 64.8 and 73.0%, respectively, and the contents of Fe2O3 and Al2O3 are below 5.9%. The sample MUGEO 2460 presents the higher values of Si, V, Cs, Dy, Er, Eu, Gd, Tb, Yb, Ho, Lu, Y, As, In, Sb, Se, Te, Cd, Co, Ni, Cu, Mo, and Pb. This sample is dominated by todorokite and cryptomelane, both (mainly todorokite) being carriers of HREE + Y and V, Cs, As, In, Sb, Co, Ni, Cu, Pb, Mo, Se, Te and Cd. The two bulk samples show very low contents of P, C and S (Table 12). The order of high magnitude of the contents of much of these elements (V, Co, Ni, Cu, Pb, Mo, Se, Te and Cd) for sample MUGEO 2460 may support a possible hydrothermal contribution and/or still hydrogenous nodules from the ocean bottom (Glasby et al., 2005; Polgári et al., 2012; Jones et al., 2013; Molnár et al., 2017) as the primary source for manganese. These chemical elements together with many others such as Mn, Fe, Mg, S, P, and so on (As, Co, Ce, Ba, Sr), also belong to bio-essential elements reported by Polgári et al. (2012) and Zarasvandi et al. (2013) in the Jurassic Mn-carbonate-bearing black shales with microbial activity from Úrkút (Hungary). Therefore, the mineralogical and chemical differences between the two stromatolite-like structures are clear, showing that the microbial activity and the continental and hydrothermal contributions changed locally, the latter being perhaps the main source of these metals. It also became clear that the mineralogy of the Mn-oxyhydroxides in turn controls the fixation of these chemical elements. The high contents of Si and Mn suggest a microbial silicium bacteria.
Table 12 – Chemical composition of two equal manganese-rich stromatolites-like structures (samples 1: ESTMn01 and 2: MUGEO 2460) from Azul mine 2 composed of distinct manganese oxides (cryptomelane-hollandite, and todorokite and cryptomelane, respectively).
| SAMPLE ID: | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | Cr2O3 | TiO2 | MnO | P2O5 | SrO | BaO | LOI | Total |
| 1.) ESTMn01 | 18.7 | 5.9 | 5.07 | 0.04 | 0.32 | 0.06 | 2.13 | 0.01 | 0.24 | 46.1 | 0.15 | 0.01 | 0.83 | 11.4 | 90.96 |
| 2.) Mugeo2460 | 24.7 | 3.95 | 1.73 | 0.04 | 0.29 | 0.07 | 0.89 | 0.01 | 0.16 | 48.3 | 0.11 | <0.01 | 0.01 | 10.3 | 90.56 |
| C | S | Ba | Ce | Cr | Cs | Dy | Er | Eu | Ga | Gd | Ge | Hf | Ho | La | |
| 1.) ESTMn01 | 0.09 | 0.01 | 7120 | 62.1 | 50 | 1.95 | 1.98 | 1.07 | 0.53 | 30.7 | 2.19 | <5 | 2.8 | 0.35 | 14 |
| 2.) Mugeo2460 | 0.09 | 0.02 | 101 | 31.6 | 40 | 2.26 | 2.74 | 1.35 | 0.62 | 30.7 | 2.39 | <5 | 1.6 | 0.55 | 12.6 |
| Lu | Nb | Nd | Pr | Rb | Sm | Sn | Sr | Ta | Tb | Th | Tm | U | V | W | |
| 1.) ESTMn01 | 0.13 | 2.7 | 13.7 | 3.92 | 54.5 | 3.09 | 1 | 70.3 | 0.2 | 0.35 | 4.61 | 0.2 | 1.65 | 55 | <1 |
| 2.) Mugeo2460 | 0.15 | 1.7 | 9.6 | 2.71 | 40.9 | 2.16 | 1 | 18.5 | 0.1 | 0.4 | 3.11 | 0.21 | 0.85 | 201 | <1 |
| Y | Yb | Zr | As | Bi | Hg | In | Re | Sb | Se | Te | Tl | Ag | Cd | Co | |
| 1.) ESTMn01 | 10.6 | 1.09 | 102 | 14.2 | 0.06 | 1.045 | 0.016 | 0.002 | 0.74 | 0.5 | 0.11 | 4.77 | <0.5 | 6.6 | 262 |
| 2.) Mugeo2460 | 13.6 | 1.13 | 58 | 17.4 | 0.06 | 0.055 | 0.019 | <0.001 | 0.85 | 3.2 | 0.27 | 0.17 | <0.5 | 22.6 | 295 |
| Cu | Li | Mo | Ni | Pb | Sc | Zn | |||||||||
| 1.) ESTMn01 | 302 | 10 | 1 | 62 | 78 | 15 | 667 | ||||||||
| 2.) Mugeo2460 | 719 | 10 | 35 | 190 | 223 | 5 | 655 |
SEM scattering images allow some microbial structures in the manganese/silicium-rich stromatolite-like structures to be individualized (Figure 18). The SEM/EDS analyses also support the Mn-bearing silicium bacteria and Mn-bearing protozoan. Zeiner et al. (2006) experimentally discussed the importance of microorganisms, such as tetrahymena thermophyla protozoa and oxidant bacteria such as leptothrix discophora SS-1, in the formation of manganese oxides on the basis of predator and prey, respectively, and concluded that the extracellular Mn-oxide coating protects cells from predators. The importance of microbial activity for fixing and oxidizing Mn in solution and fixing in sediments, is widely discussed and has been recognized in manganese deposits from Paleoproterozoic to the Recent (Yu et al. 2016; Shiraishi et al., 2016; Sinisi et al., 2018).
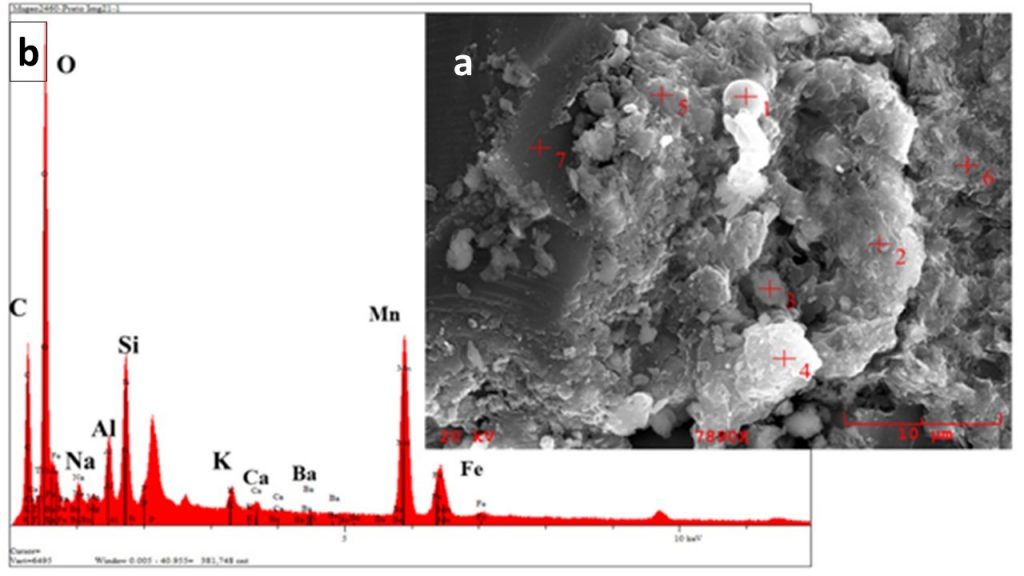
Figure 18 – (a) SEM images from manganese oxyhydroxides showing possible microbial structures (point analyses: 1, 2, 3, 4, 5, 6 and 7) in the manganese-rich stromatolite-like structures of the sample MUGEO 2460. At point 7 possible Mn-bearing silicium bacteria and at point 1 possible Mn-bearing protozoarian. (b) EDS spectrum for Mn-protozoan (analysis point 1).
After the micromorphology and micrometre chemical analyses, we interpret these possible microbes as being equivalent to a silicium bacterium, bacterial colonies (moulds, bulbous and micro-nodules) and locally fungi-like based on Nayak et al. (2013), Rajabzadeh et al. (2017) and Park et al. (2018). All of the analysed points show the presence of carbon, reaching up to 38%, with silicon ranging from 5 to 52% (point 7 manganese from 0.8 to 45.8%, potassium from 0.10 to 1.70% and barium, 0.19%, in addition to Fe, Al, Ti, P and Na, and of course, oxygen (Table 13). As the contents of Mn and K (?) increase, those of Si and C decrease, suggesting a capture of Mn and K by exchange with Si and C, while those of oxygen practically do not vary (Figure 19). These chemical elements are the major chemical components of the manganese ore of Azul. Similar EDS chemical analyses of Mn-bearing microbes are discussed by Nayak et al. (2013), as well as by Biondi & Lopez (2017) for several microbialites related to kremydillites, some of them rich in C, Si and Mn. The marked presence of carbon and features suggestive of microbial activity leave no doubt as to the participation of microorganisms in the fixation and deposition of manganese oxides in the Azul deposit during Early Proterozoic, at least partially, similarly to most manganese deposits around the world (Roy, 2006; Polgári et al., 2012, 2016; Kuleshov, 2017; Rajabzadeh et al, 2017; Molnár et al., 2017; Biondi and Lopez, 2017; Biondi et al., 2020).
Table 13 – SEM/EDS chemical analyses (Wt. % on 100% basis) of probable protozoan and bacteria and some carbonaceous records in the Mn-bearing stromatolite-like structure (Sample MUGEO 2460) from Azul manganese deposit. Point analyses on the SEM image are indicated in the figure (Figure 18). nd: not detected.
| Elements | Point 1 | Point 4 | Point 3 | Point 7 | Point 2 | Point 5 | Point 6 |
| Mn-rich Si bacteria/ protozoan + COM | Mn-bearing | Mn-bearing | Silicium bacteria | Mn-rich Silicium bacteria | Mn-rich Silicium bacteria | Mn-rich Silicium bacteria | |
| Silicium bacteria + COM | Silicium bacteria + COMmatter | ||||||
| Carbon | 23.75 | 38.37 | 16.92 | 1.12 | 3.12 | 2.55 | 2.24 |
| Oxygen | 39.02 | 39.55 | 44.17 | 44.63 | 35.94 | 34.61 | 32.39 |
| Silicium | 5.10 | 9.86 | 20.74 | 52.18 | 7.57 | 14.34 | 6.85 |
| Aluminum | 2.46 | 3.24 | 2.79 | 0.55 | 5.34 | 4.36 | 5.18 |
| Manganese | 20.43 | 5.56 | 13.04 | 0.80 | 43.09 | 40.34 | 45.80 |
| Iron | 5.33 | 0.67 | 0.61 | 0.12 | 1.93 | 1.45 | 4.38 |
| Magnesium | 0.24 | 0.31 | 0.27 | 0.03 | 0.37 | 0.42 | 0.55 |
| Potassium | 0.96 | 0.99 | 0.79 | 0.10 | 1.70 | 1.20 | 1.50 |
| Barium | 0.07 | nd | 0.13 | 0.17 | 0.08 | 0.11 | 0.19 |
| Sodium | 1.11 | 0.39 | 0.12 | nd | 0.24 | 0.07 | Nd |
| Phosphor | 1.12 | 0.55 | 0.27 | 0.22 | 0.44 | 0.40 | 0.50 |
| Titanium | 0.06 | 0.07 | nd | nd | 0.06 | nd | 0.38 |
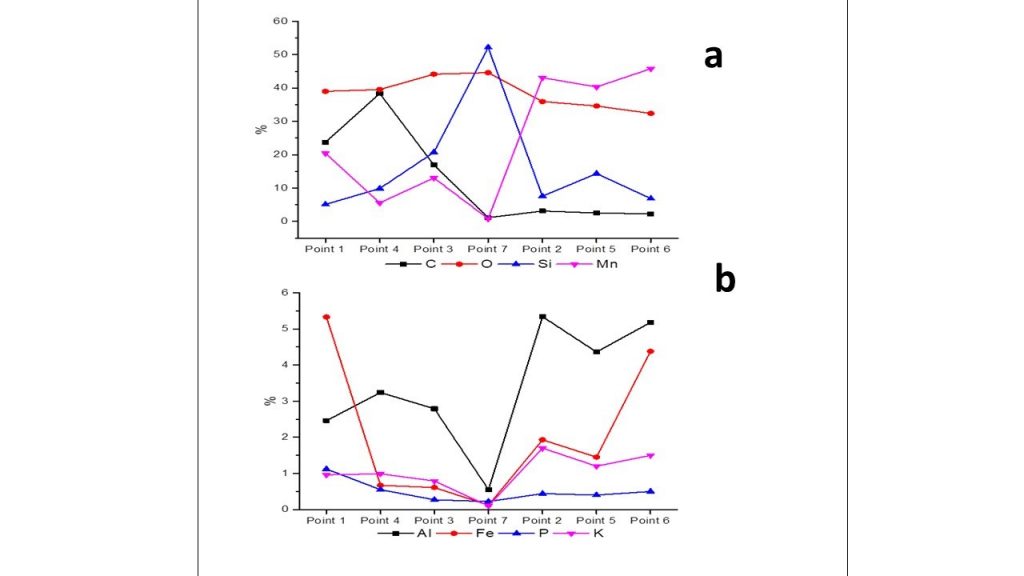
Figure 19 – The chemical composition variation at the distinct microbial features (Siliceous carbonaceous matter comparable to described by Biondi & Lopez, 2017) obtained by SEM/EDS in the sample MUGEO 2460. (a) C-O-Si-Mn, the major components and (b) Al-Fe-P-K, the minor components, after Table 13. The analyses of points 1, 2, 5 and 6 showing display Mn-K positive and Si negative correlated.
Fault-Hosted Manganese Ore: Tectonic Mineralization – The Trans-Amazonian tectonic deformation, which reactivated the Archean Carajás fault, seems to have hit the manganese-rich sediments of Azul right at the end of its sedimentation. However, north-south and northwest-southeast faults and shear zones supposedly correlated to the Brazilian tectonic deformation Cycle overprinted the entire sequence (Araujo & Sousa, 2018). They developed intense cracking, micro faults, folds and micro folds, whose voids were infilled with pyrolusite, manganite and perhaps nsutite, being massive to crystalline, with high manganese ore content. Structural controls of fault-hosted manganese are noticed at Woodie Woodie, East Pilbara, Australia (Jones et al., 2013), Hotazel formation, Kalahari manganese field, South Africa (Gutzmer and Beukes, 1996; Roy, 2006; Vafeas et al, 2018).
Kaolin Veinlets and Bedding Laminae – The grey shales and the dark grey manganese-bearing shales and manganese ores were strongly affected by pockets and venules of white kaolin in almost all of their extensions and especially in zones of greater tectonic deformation represented by faults, folds and shear zones. In general, these venules may contain isolated crystals from sulphides, commonly pyrite, and rhodochrosite. The main mineral of kaolin venules is obviously kaolinite, which is microcrystalline, in well-formed crystals, such as platelets or stacking of pseudo-hexagonal platelets, at the level of almost booklets, with no preferred direction (Figure 20). The chemical analyses by EDS confirm the chemical composition for kaolinite, and the picks for Mn and K show their relationship with the hosting ore represented by cryptomelane. These characteristics of kaolinite and of the fault-hosted Mn-oxyhydroxides that were shown earlier, reinforce the significant range of the tectonic deformation related to faults and fault zones, mainly.
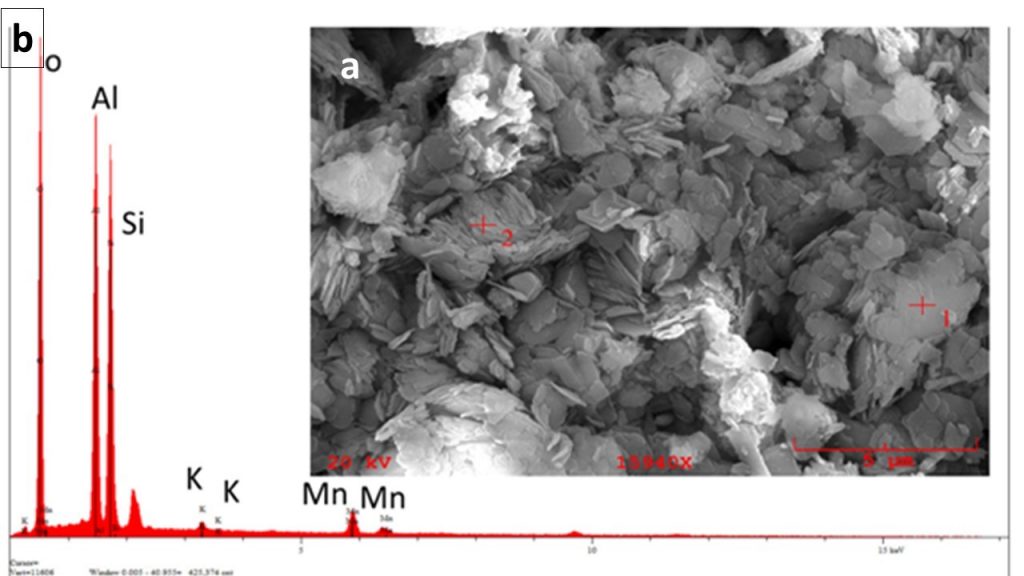
Figure 20 – (a) SEM images of kaolinite crystals coming from kaolin veinlets in gray shales and red siltstones and (b) EDS spectrum showing the composition of kaolinite (Si, Al, and O) and some contents of cryptomelane (K, Mn and O).
Major and Trace Element Contribution –The chemical composition of the manganese-bearing shales, when compared to some world-class deposits, in terms of K, Mg, Ca and Ti, are similar to hydrothermal and/or hydrogenous nodules, modern deposits, and the Chinese Neoproterozoic (Table 14). Alternately, the low levels of Na correlate with the giant deposits of Kalahari (Maynard, 2004), the metasedimentary manganese (Late Archean) of Western Dharwar Craton, southern India (Sethumadhav et al., 2010), and Urucum (Viehmann et al., 2016). Based on the Al2O3 and SiO2 concentrations, the Mn-rich Azul sediments and ore would involve both normal and abyssal sedimentation (Figure 21 A). As shown in the diagram of Si-Fe-Mn (Figure 22 A), the Mn-bearing black shales do not plot in any of the main source Mn field but come closer to hydrogenous.
Table 14 – Chemical composition of some world important manganese deposits and compared with Past Archean Shales. Modified after Maynard (2004).
| Chemical | Kalahari
S.Africa |
Tanganshan
China |
Xiangtan
China |
Molango
Mexico |
Hydrothermal
Pacific |
Hydrogenous
Pacific |
Hydrogeneous
Nodules |
Post
Archean |
||
| Elements | Units | Paleoprot. | Neoprot. | Neoprot. | Jurassic | Modern | Modern | Modern | shales | |
| Mn | % | 36.5 | 44.9 | 42.3 | 28.0 | 37.0 | 22.1 | 18.6 | MnO | 0.11 |
| Fe | % | 4.22 | 2.66 | 1.54 | 8.53 | 1.87 | 15.1 | 12.5 | FeO | 6.5 |
| Na | % | 0.01 | 0.03 | 0.01 | 0.00 | 2.37 | 1.6 | 1.7 | Na2O | 1.2 |
| Mg | % | 1.93 | 1.09 | 1.23 | 5.10 | 1.95 | 1.26 | 1.6 | MgO | 2.2 |
| Al | % | 0.09 | 0.74 | 0.44 | 1.43 | 1.57 | 1.01 | 2.7 | Al2O3 | 18.9 |
| Si | % | 2.40 | 1.54 | 4.75 | 5.36 | 7.73 | 3.69 | 7.7 | SiO2 | 62.8 |
| P | % | 0.01 | 0.12 | 0.07 | 0.06 | 0.13 | 1.18 | 0.25 | P2O5 | 0.16 |
| K | % | 0.00 | 0.01 | 0.17 | 0.00 | 1.01 | 0.56 | 0.7 | K2O | 3.7 |
| Ca | % | 12.2 | 2.72 | 1.34 | 1.73 | 2.48 | 4.13 | 2.3 | CaO | 1.3 |
| Ti | % | 0.01 | 0.14 | 0.10 | 0.06 | 0.15 | 0.77 | 0.67 | TiO2 | 1.0 |
| C organic | % | 2.90 | 0.60 | |||||||
| C carbonate | % | 3.15 | 8.70 | 8.70 | 0.1 | |||||
| d13Corg | per mil | -27.3 | ||||||||
| d13Corg | per mil | -9.1 | -13.1 | |||||||
| d18Ccarb | per mil | -10.4 | -3.6 | |||||||
| S total | % | 0.04 | 2.00 | 0.80 | 0.20 | 0.06 | 0.305 | 0.47 | ||
| d34S | per mil | 26.7 | 52.5 | 3.2 | ||||||
| Sc | ppm | 13 | 12 | 2.12 | 3.8 | 6.4 | 10 | 16 | ||
| V | ppm | 6 | 80 | 53 | 67 | 225 | 515 | 500 | 150 | |
| Cr | ppm | 16 | 23 | 24 | 15 | 48 | 22 | 35 | 110 | |
| Co | ppm | 50 | 64 | 44 | 132 | 72 | 6400 | 2700 | 23 | |
| Ni | ppm | 19 | 45 | 24 | 60 | 287 | 5400 | 6600 | 55 | |
| Cu | ppm | <5 | 16 | 11 | 7 | 228 | 1080 | 4500 | 50 | |
| Zn | ppm | 74 | 19 | 266 | 48 | 238 | 680 | 1200 | 85 | |
| As | ppm | 48 | 34 | 31 | 33 | 165 | 140 | |||
| Se | ppm | 2.0 | 0.5 | 0.12 | 0.4 | 0.6 | ||||
| Rb | ppm | 1 | 1 | 14 | <0.2 | <0.2 | <0.2 | 17 | 160 | |
| Sr | ppm | 146 | 69 | 101 | 40 | 555 | 1210 | 830 | 200 | |
| Y | ppm | 5.3 | 41 | 33 | 8.6 | 17 | 166 | 150 | ||
| Zr | ppm | 2 | 33 | 29 | 15 | 23 | 172 | 560 | 210 | |
| Nb | ppm | <0.1 | 25.0 | 17.5 | 1.5 | 9.9 | <0.1 | 50 | 19 | |
| Mo | ppm | <2 | 16.7 | 6.5 | 2 | 327 | 445 | 400 | 1.0 | |
| Sn | ppm | <1 | 6.5 | 4.0 | <1 | 2 | 4.0 | |||
| Sb | ppm | 14 | 2.8 | 1.1 | 25 | 24 | 40 | |||
| Ba | ppm | 360 | 50 | 259 | 45 | 1380 | 1700 | 2300 | 650 | |
| Pb | ppm | 6 | 68 | 8 | 6 | 45 | 1780 | 900 | 20 | |
| Bi | ppm | 0.5 | <0.1 | 7.0 | 0.25 | |||||
| Th | ppm | 0.4 | 3.3 | 1.2 | 1.4 | 0.7 | 33.0 | 30.0 | 14.6 | |
| U | ppm | 0.2 | 0.9 | 0.7 | 1.6 | 2.1 | 9.6 | 5.0 | 3.1 | |
| La | ppm | 3.18 | 32.0 | 26 | 11.8 | 18.9 | 202 | 157 | 38 | |
| Ce | ppm | 3.3 | 98.8 | 79.4 | 18.1 | 16.3 | 1100 | 530 | 80 | |
| Pr | ppm | 0.5 | 8.0 | 6.7 | 2.4 | 106 | 36 | 8.9 | ||
| Nd | ppm | 2.1 | 32.8 | 27.8 | 9.5 | 7.2 | 162 | 158 | 32 | |
| Sm | ppm | 0.33 | 7.60 | 5.50 | 1.70 | 0.99 | 42 | 35 | 5.6 | |
| Eu | ppm | 0.16 | 2.35 | 1.14 | 0.43 | 0.28 | 9.90 | 9.00 | 1.1 | |
| Gd | ppm | 0.58 | 7.52 | 5.56 | 1.87 | 26 | 32 | 4.4 | ||
| Tb | ppm | 0.09 | 1.24 | 0.88 | 0.29 | 0.25 | 7.53 | 5.40 | 0.77 | |
| Dy | ppm | 0.55 | 7.99 | 5.85 | 1.49 | 57.8 | 31.0 | 4.4 | ||
| Ho | ppm | 0.14 | 1.55 | 1.23 | 0.31 | 6.60 | 7.00 | 1.0 | ||
| Er | ppm | 0.42 | 4.54 | 3.28 | 0.85 | 31.9 | 18.0 | 2.9 | ||
| Tm | ppm | 0.06 | 0.58 | 0.46 | 0.11 | 4.30 | 2.30 | 0.40 | ||
| Yb | ppm | 0.45 | 3.50 | 2.75 | 0.75 | 0.78 | 17.7 | 20.0 | 2.8 | |
| Lu | ppm | 0.08 | 0.43 | 0.38 | 0.15 | 0.14 | 3.34 | 1.80 | 0.43 | |
| Sources | a,b | a | a | a,c,d | e | e | F | g | ||
This chemical composition is compatible with mineralogy dominated by quartz and illite/sericite, and partly chlorite, in addition to pyrite. TiO2 levels are higher than in manganese black shales. The near absence of CaO is remarkable, which denotes the absence, for example, of calcite and/or dolomite, common in Mn-rich carbonate rocks (Gutzmer and Beukes, 1996). They can be correlated to the Post Archaean Shales, but they are impoverished in Ca and Na, mainly, thus showing a certain partial signature with the manganiferous shales. These shales in the Azul deposits, besides Mn, differ markedly in term of the higher contents of Si, Al, and Ti, thus demonstrating their greater contribution in terrigenous material (quartz, illite/sericite and kaolinite).
As regards Co, Ni, Cu and Zn, as well as As, Mo, Sb and Ag, their levels in the manganese-bearing shales allow for comparison with the hydrothermal-type Mn-oxyhydroxide deposits, mainly and in part to the modern hydrogenous nodules. In the diagrams of (Co+Ni+Cu)*10-Fe-Mn and Zn-Ni-Co (Figures 22 B and C) they truly fall in the field between hydrogenous and hydrothermal sources, suggesting both metal sources for Mn in Azul, as already demonstrated by aluminosilicate minerals. The Neoproterozoic Datangpo manganese deposit in China, after analysis of these elements, shows a hydrogenous source with hydrothermal contribution after other geochemical proxies, for instance, REE (Yu et al., 2016), while the Mesozoic of Iran hydrothermal (Rajabzadeh et al., 2017) and those of Úrkút, Hungary, are typically hydrothermal and diagenetic bacterial (Polgári et al., 2012).
The contents of REE, in turn, allow us to confront the manganese shales partially with the Neoproterozoic deposits of China, far from the classic Kalahari deposit (Maynard, 2004, Table 10). In turn, Ba, Pb and Sr reinforce the relationship with the hydrothermal and modern hydrogenous deposits. Likewise, the values of Zr, Y, Th, Uand V, however far from Kalahari and Neoproterozoic China deposits (Table 14). In the REE – Al2O3 diagram (Figure 21 B), the sediments and Mn ores of Azul plot mainly as terrigenous input, microorganism enrichment and Mn-oxyhydroxides absorption. This reinforces what has already been demonstrated, strengthening the importance of the cryptomelane-hollandite, todorokite and birnessite, sedimentary precursor and early diagenesis formed.
The carbonaceous dark shales and dark grey to black shales sedimentary rocks chemically correspond to black shales, normal shales and carbonaceous shales (Taylor & McLennan, 1985). The manganese-rich shales are distinguished by the much higher values of Co, Ni, Cu, Zn, Ba, Pb, Sr and in part Ce (Figure 23). The high values of these elements demonstrate that the source of much of them would have been hydrothermal sources, as shown by comparisons with modern ocean deposits and modern hydrogenous deposits (Table 11) and in the previous diagrams (Figures 21 B and C). In turn, the great volume of siltstones and red sandstones demonstrate, at the same time, a great terrestrial, continental contribution. In general, the Mn-rich black shales and the manganese ore of Azul are relatively enriched, and the Mn-poor dark shales are impoverished. They range from relatively low-REE-content Post-Archean Shales to rich-REE-content hydrogenous deposits (Table 11), much higher than the REE contents of Paleoproterozoic biotite schists and graphite schists associated with Mn-carbonate and silicates marbles at Serra do Navio manganese deposits, considered to be derived from black shales and siltstones that are locally carbonaceous (Chisonga et al., 2012), and much higher than Mesozoic manganese mineralizations in the Abadeh-Tashk area (Rajabzadeh et al., 2017).
The distribution of REE in Mn-rich Azul rocks and ores normalized to PAAS sediments (Figure 24) displays a slightly positive Ce and Eu anomaly with middle rare earth subtle enrichment similarly to Neoproterozoic Dapangto Mn deposits in China; they differ only in concentration, which is higher in Azul Mn ores. A similar pattern showed the Archean black shales REE associated with banded iron formation in Carajás, however chondrite-normalized, displaying a hydrothermal signature in an anoxic and euxinic marine environment (Cabral et al., 2013). This differs from what was observed in the Mn and Fe Neoproterozoic deposits of the Urucum/Santa Cruz, which have a strong negative Ce anomaly (Klein and Ladeira, 2004; Angerer et al., 2016 and Biondi & Lopez, 2017). After Klein and Ladeira (2004) the general trend of the REE NASC-normalized plots showing some enrichment of the heavy REE can be well compared to that of modern seawater, concluding that the Fe, Mn, and Si is from typical ocean water with some deep-sea hydrothermal input. Alternately, these behaviour patterns of REE with a positive Ce anomaly (Figure 24) are consistent with those of the phosphatized Fe-Mn nodules, hydrogenetic Fe-Mn nodules and hydrogenetic Fe-Mn crusts (Xiao et al., 2017), which again differ in concentration, with it being higher in Azul. This suggests that Mn and Fe oxyhydroxides are also deposited as amorphous and poorly crystalline phases on a solid or accretion substrate, very slowly, initially sediment-free, Fe-bearing vernadite (d-MnO2) and FeOH, respectively (Xiao et al., 2017), usually as colloidal particles with a surface charge, slightly positively surface-charged Fe oxides and negatively surface-charged Mn oxides (Xiao et al., 2017). This must have happened in Azul’s manganese deposit, similarly to Neoproterozoic Datangpo-type sedimentary manganese deposits in Guizhou Province, China (Xiao et al., 2017), which contrary to Azul, went into Mn carbonate type on later diagenesis. It is well known that the Mn-oxyhydroxides have a capacity to scavenge several trace elements from sea-water (Bau and Koschinsky, 2009). The wide presence of cryptomelane demonstrates intense diagenetic activity, with interaction between the surface-charged amorphous Mn-oxides and cations, such as of the elements K, REE, Co, Ni, Cu, Zn, and so on. Ba++ seems to have come from percolating fluids along fractures and faults, forming hollandite. Some samples with positive Ce anomalies perhaps represent the strong oxidizing conditions that favoured the Ce oxidation and even the discrete formation of cerianite terrestrial weathering, before coming to Azul basin.
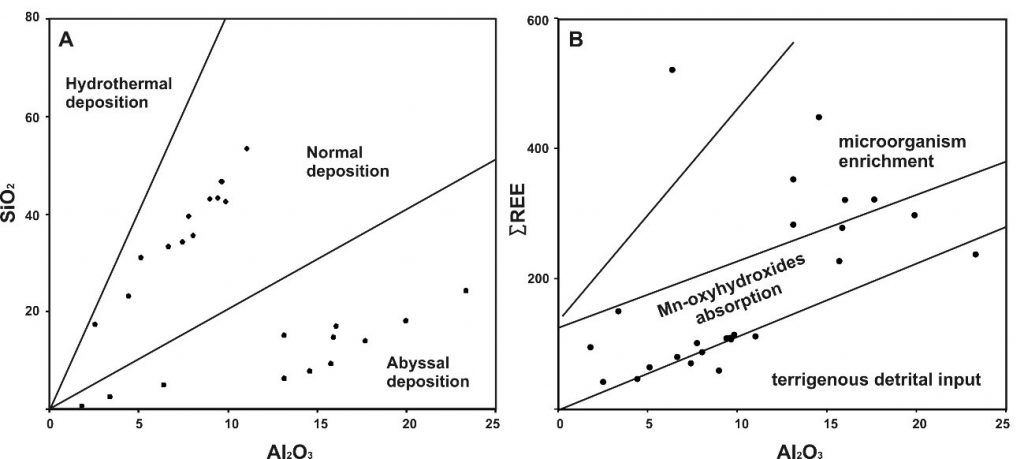
Figure 21 – The chemical binary plots SiO2-Al2O3 (A) and ƩREE – Al2O3 (B) for Mn-ore and Mn-bearing black shales in Azul. Adapted to Xiao et al (2017).
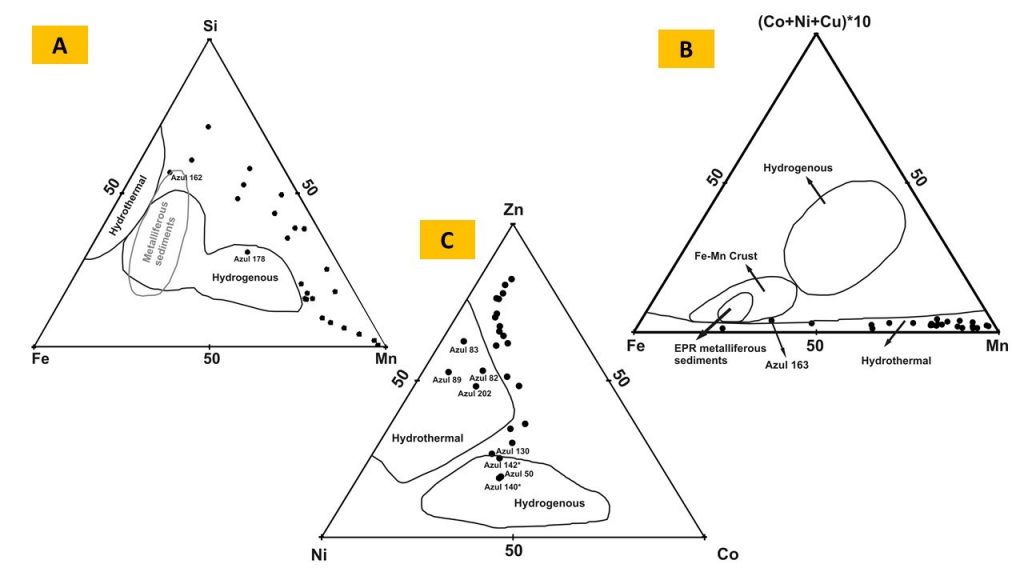
Figure 22 – Plots of the chemical data obtained from Azul manganese ores and black shales in distinct diagrams for interpret possible Mn source and environment formation: (A); In the diagram Si-Fe-Mn indicated for classification marine Fe-Mn deposits (Reolid et al, 2011); (B) In the diagram (Co-Ni-Cu) *10-Fe-Mn after Bonatti et al., 1972; (C) Plots in the diagram Zn-Ni-Co after Hein et al (1997).
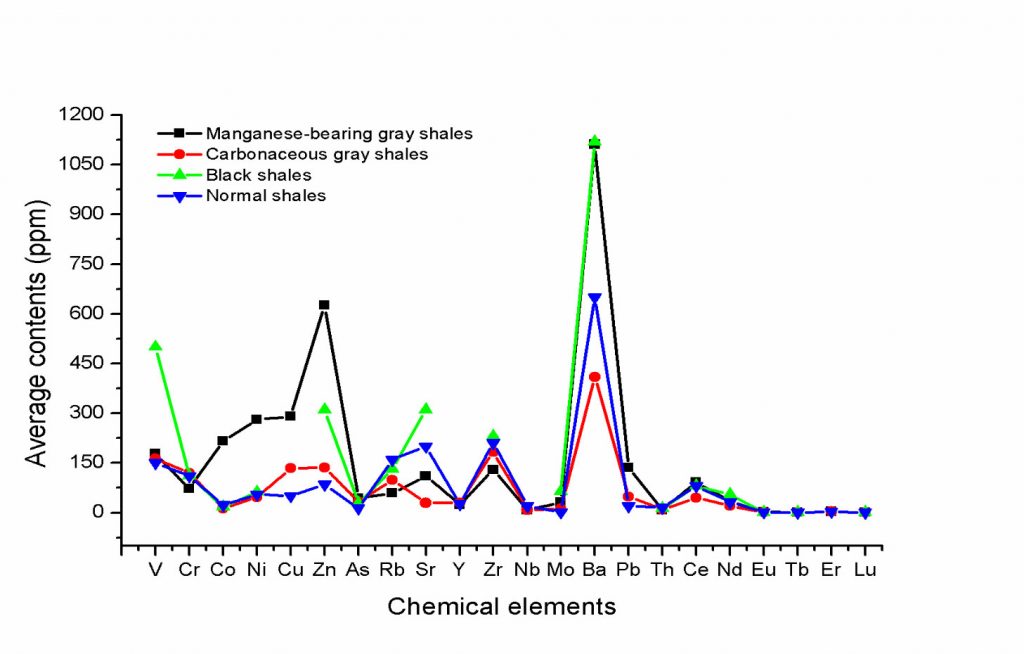
Figure 23 – Distribution of REE PAAS-normalized for Mn-ores and Mn-bearing black shales in Azul compared to distinct genetic features of modern marine ferromanganese oxyhydroxides precipitates proposed by Bau et al. (2014).
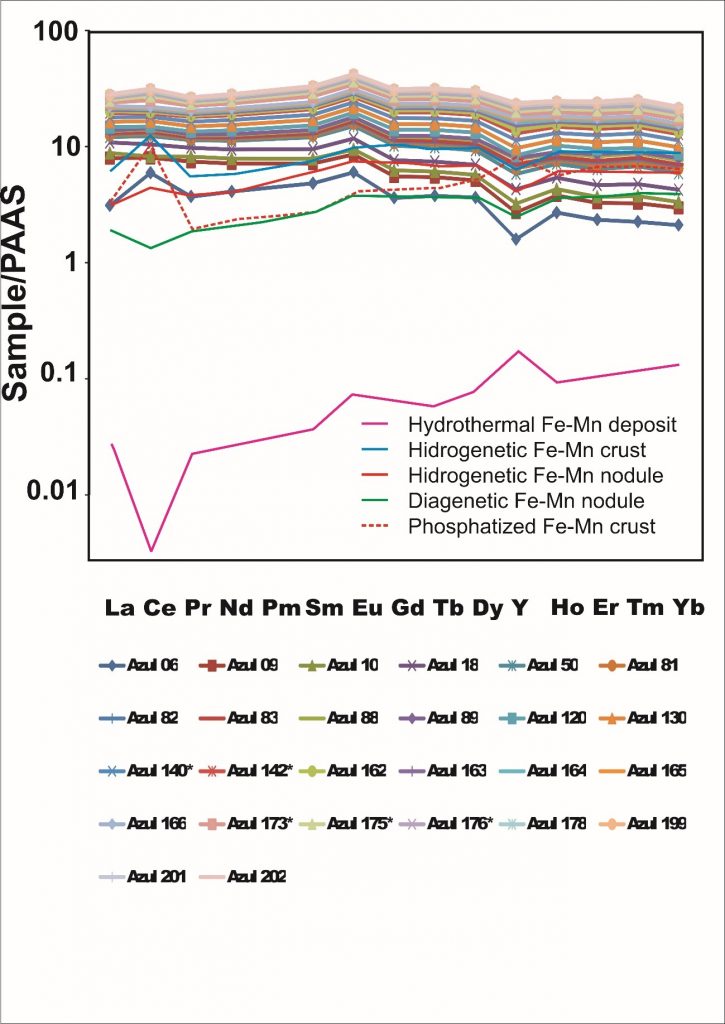
Figure 24 – Comparison between the dark manganese-rich grey shales mineralized from Azul with some similar sediments, for instance, black shales and carbonaceous and normal shales (Taylor & McLennan, 1985).
Isotopic Composition for d 13C and d 18O in Mn-Carbonates – The d 13C values of the Azul Mn carbonates (Table 15) are equivalent to those of modern Baltic Sea deposits (d 13C per mil PDB), which are richer in Mn and poorer in Ca and Mg (Maynard, 2004), indicating an environment linked to shallow and near-shore sediments.
The isotopic compositions d 13CPDB and d 13O SMOW of the Azul Mn-carbonates (mainly rhodochrosite), slightly depleted in d 18O SMOW, demonstrate a formation during an earlier diagenesis (Table 15) (Figure 25) by comparison to Paratethys deposits (Kuleshov, 2003; 2017). Microbially mediated reactions between manganese oxide and organic matter promote the formation of the manganese carbonate in the deposits (Kuleshov, 2017; Yu et al., 2019). This mechanism also resulted in the negative δ13C signals preserved in the Paleoproterozoic manganese deposits (Kalahari, Nsuta, Katanga and Moanda and Serra do Navio (Putter et al., 2018); Neoproterozoic manganese deposits, Datangpo in China (Yu et al., 2017, 2019) and Mesozoic Úrkút in Hungary (Polgári et al., 2012; Molnár et al., 2017). In these conditions, the amorphous and poor crystalline (Mn,Fe)-oxyhydroxides remain partially and an expressive part of the organic matter laminae, stromatolite-like structure (Molnár et al., 2017). This stromatolite-like structure has also been locally observed in the Azul manganese deposits. In the Serra do Navio manganese deposits, besides the positive Ce anomalies, the δ13C PDB values of −4.3 to −9.4 per mill suggest that Mn-carbonates derived during suboxic diagenesis from sedimentary Mn(IV) oxyhydroxide precipitates (Chisonga et al., 2012).
Even if present, rhodochrosite is not an abundant mineral in the Azul manganese deposits, although it has been described as forming two manganese units in a single drill hole carried out in the early geological explorations. The presence of diagenetic carbonates presupposes that of a carbonaceous organic matter. However, the Mn deposits of Azul still have organic carbonaceous material, but the restricted formation of rhodochrosite, suggests that these deposits did not stay for a long time below the redoxcline surface (water/sediment interface), which would favour the reduction of Mn and oxidation of organic matter (Molnár et al., 2017).
Table 15 – Values of isotopic composition for d 13C e d 18O in manganese carbonates from Azul deposit. d 18Osmow = 1.03091. d16OPDB + 30.01.
| Sample ID: | d13C PDB | d18O PDB | d18O SMOW |
| AZUL 83 | -11.29 | -5.97 | 23.85 |
| AZUL 88 | -11.14 | -4.6 | 25.27 |
| AZUL 89 | -12.31 | -5.64 | 24.19 |
| AZUL162 | -16.24 | -7.32 | 22.46 |
| AZUL165 | -12.11 | -5.35 | 24.49 |
| AZUL166 | -12.56 | -5.94 | 23.89 |
| AZUL201 | -8.99 | -5.26 | 24.59 |
| MF-14 | -12.33 | -5.41 | 24.43 |
| MF-22 | -11.74 | -5.42 | 24.42 |
| MF-24 | -12.27 | -5.26 | 24.59 |
| Average | -12.10 | -5.617 | 24.018 |
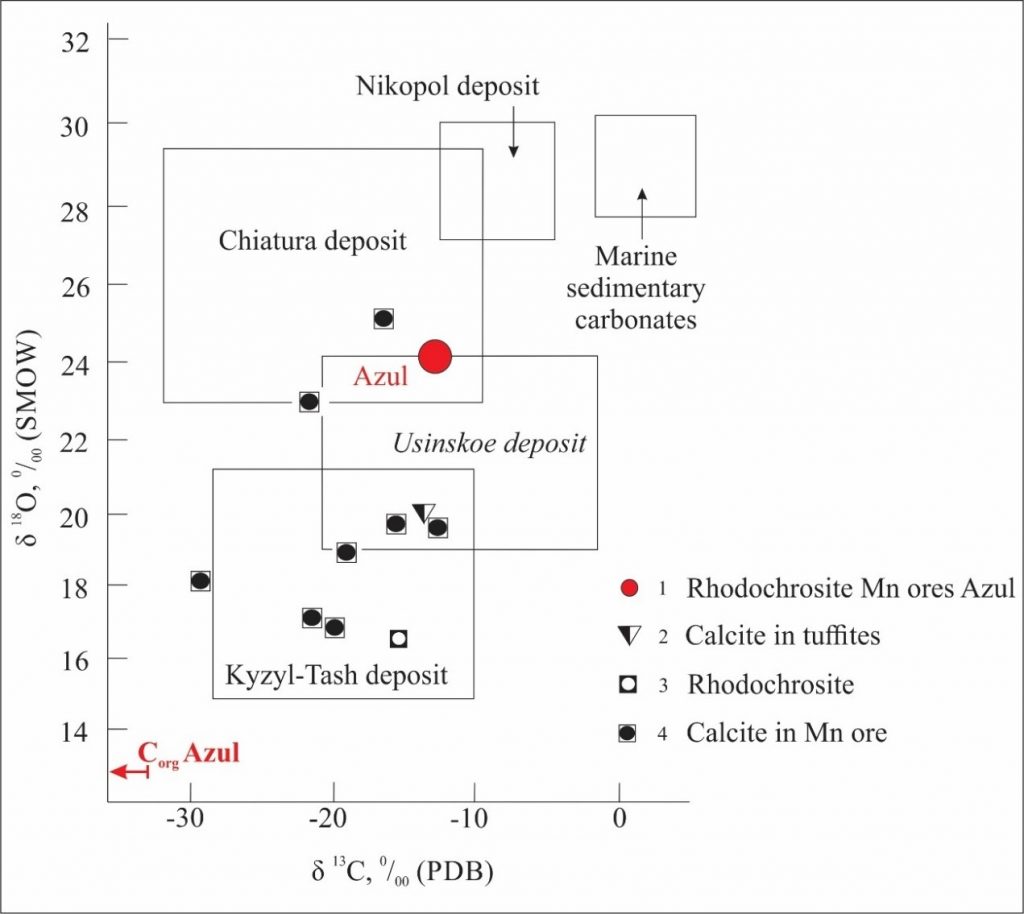
Figure 25 – Diagram for delta δ13C per mil PDB vs δ18O per mil SMOW for distinct manganese deposits, inclusive Azul and marine sedimentary carbonates and δ13Corg for Azul organic matter from carbonaceous shales. Modified after Kuleshov (2017).
δ13C PDB for Organic Matter – The values of δ13C PDB for organic matter contained in the grey shales associated with the undisturbed red siltstones show strong depletion (Table 16), a geochemistry signature found in the Azul black shales and in other similar manganese sedimentary deposits. Stable δ13CPDB isotope analyses presented almost constant δ13CPDB values (32.8‰ through 34.6‰, N=10) in the range of the numerous values of the Paratethys Early Oligocene manganese deposits, which vary from 25 to 35 ‰ (Lan et al., 2019; Kuleshov, 2003, 2017); the Vendian-Cambrian, North Iran, which shows high concentrations of Mn, P and Ba and high abundances of fossil phytoplankton associated with black shale (Kimura et al., 1997); the organic carbon-rich sediments in southern high latitude deep sea in the range of 16.5 through 28.7‰ (Sackett, 1986); and those of the Molango Mn deposits in Mexico (Maynard, 2004). Negative shifts in the δ13C values have been interpreted to reflect recycling of respired CO2 in a more localized reservoir (Sageman, 2004), with OM probably being deposited contemporaneously with the Mn-oxyhydroxides in Azul´s manganese sedimentary environment.
Meanwhile, the negative and practically homogeneous δ13C PDB values in the sediments of the manganese deposits in Azul suggests homogeneity in the source of organic matter, therefore without isotopic fractionation. Part and locally, this OM was oxidized and allowed the formation of Mn-carbonate, by reducing the Mn of the oxyhydroxides, during the diagenesis, demonstrated by the formation of rhodochrosite strictly.
Table 16 – Values of isotopic composition for δ13C PDB for organic matter from carbonaceous shales.
| Samples ID | δ 13C PDB |
| AZUL 153 | -33.5 |
| AZUL 155 | -33.9 |
| AZUL160 | -34.1 |
| AZUL161 | -33.1 |
| AZUL162 | -32.8 |
| AZUL164 | -33.4 |
| AZUL165 | -34.1 |
| AZUL166 | -33.3 |
| AZUL199 | -34.6 |
| AZUL 201 | -34.2 |
| Average | -33.7 |
δ 34S Pyrite Isotopic Composition – The values of the isotopic composition δ 34S of pyrite from grey and dark grey shales varied between 3.85 and 8.54 per mill (Table 17), in the range of the Archean (2.7 Ga) black shales associated with BIF in Carajás, Brazil presented by Cabral et al (2013, 2017). The δ34S-isotope values range between +0.3 and +10.7‰, suggesting microbial sulphate reduction. The Fe and S isotope analyses carried out in pyrites contained in Águas Claras sandstones by Fabre et al. (2011) showed a mean value of δ34S = 11.97 ‰, which is only slightly above that for the black shale pyrites analysed here. They conclude that a near-complete reduction of sulphate could be expected in the sediment and would result in a δ34S signature for the diagenetic pyrites nearly identical to the signature of seawater sulphate. The value δ34S = 11.97 ‰ measured by Fabre et al. (2011) is close to the range in δ34S values that was suggested as representative for Paleoproterozoic seawater sulphate approximately 2.1 Ga ago (Schröder et al., 2008). These values are low and are compatible with the atmosphere of the Paleoproterozoic or even Archean because atmospheric oxygen was too low, insufficient to oxidize the reduced sulphur to form sulphate in seawater (Goldhaber, 2004), a possible source of sulphur for Mn-rich carbonaceous shales of Azul deposits. This slightly higher isotopic composition allows us to speculate regarding the increase of atmospheric oxygen already in the Proterozoic approximately 2.1 Ga, the age of the Águas Claras Formation, which carries the manganese mineralization. It also strengthens the idea that sulphides, such as pyrite, are of late diagenetic origin, certainly linked to the partial oxidation of organic matter to the partial formation of carbonates, such as rhodochrosite in areas affected by microfractures and fissure venules of pyrite (pyrite + rhodochrosite/calcite + quartz). Scott et al. (2014) by using pyrite sulphur-multiple isotopes from Proterozoic black shales, found a large range in the δ34S and concluded that there is evidence for rapid expansion and contraction of the early Paleoproterozoic seawater sulphate reservoir, with the rising of seawater sulphate (Schröder et al., 2008) expanding during the Great Oxidation Event ca. 2.3 Ga and contracting at 2.06 Ga, just close to Águas Claras sedimentation
Table 17 – Values of isotopic composition for δ 34S in pyrites from dark shales in Azul manganese deposit.
| Samples ID | δ 34S |
| AZUL155 | 3.85 |
| AZUL158 | 6.43 |
| AZUL161 | 8.17 |
| AZUL162 | 7.79 |
| AZUL164 | 8.54 |
| AZUL165 | 8.25 |
| AZUL199 | 6.90 |
| Average | 7.13 |
Remarks on the Environmental Azul Sedimentary Manganese Deposits – The Azul manganese ore deposits were deposited probably as amorphous or low crystallinity Mn(IV)-oxyhydroxides on a shallow marine platform with great production of carbonaceous organic matter and some microbial activity, which in part allowed the formation of Mn-rich stromatolite-like structures, therefore above the redoxcline line. Sedimentation occurred during the Paleoproterozoic approximately 2.1 Ga when microbial activity promoted certainly the oxidation of dissolved Mn(II) in marine water. The stromatolite-like structures indicate shallow and flat deposition ambience. The various black shale lenses carrying Mn-oxyhydroxides located in different lateral and vertical positions and displaying variable lengths, suggest micro-basin formations favourable to the precipitation of Mn oxyhydroxides over time and space. The sedimentation was accompanied by a large terrestrial contribution of sediments with oxidized iron minerals (oxides) indicated by thick and large packets of reddish mudstones/siltstones and sandstones. The major Mn source comes from continents (hydrogenous) indicated by chemical composition diagrams as oxyhydroxides supporting a deep chemical weathering taking place at the time of the sedimentation or soluble and then oxidized via microorganism, for deposition of amorphous Mn-oxyhydroxides. However, a fraction of Mn also received hydrothermal contributions, in part in abyssal deposition, as suggested by the chemical diagrams and indicated by high concentrations of Ba, Cu, Ni and Co, in addition to high concentration of REE. During the diagenesis, in part probably mediated by carbon-silicium bacteria, cryptomelane and other Mn-oxyhydroxides were formed. Chlorite and pyrite were also formed, certainly also mediated by bacteria, reducing restrictive conditions, similarly for rhodochrosite, which demonstrates that the microbiological reducion activity was local, which is also indicated by the presence of carbonaceous organic matter associated with Mn-oxyhydroxide ore, as in typical early diagenesis. The lower constant negative d13C isotopic data of carbonaceous matter account for its deposition contemporaneously with the Mn-oxyhydroxides. The isotopic composition of carbonates shows that rhodochrosite, in the same manner as most deposits, is typically diagenetic, depicted by d 13CPDB and d 13O SMOW isotopic composition. At the time of early diagenesis reactions, there was low oxygen availability in the environment because the oxidation of carbonaceous organic matter had been reduced, as well as the sulphate and iron, apparently, under an oxic marine water indicated by pyrite framboids, certainly microbial mediated. This is in part confirmed by low positive δ34S-isotope values in pyrite.
However, these Mn-mineralized sediments were greatly affected during the Brazilian Tectonic Cycle that occurred in the Neoproterozoic. Most of the hollandite belongs to this phase but may still have an early diagenesis component. As a consequence, probably sericite, perhaps chlorite (anchimetamorphism), and innumerable kaolin, pyrite and other sulphides and rhodochrosites have also been developed. In addition, Mn oxyhydroxides were remobilized and re-precipitated in faults, fractures and pockets, usually as pyrolusite, forming high-grade ore locally. The association of OM + Mn-oxyhydroxides (mainly cryptomelane) + pyrite + illite/sericite ± chlorite) shows that the sedimentary sequence represented by the dark grey to black shales embedded in the primary striped red siltstones to sandstones shows the superposition of the different geological events and processes (deposition, early diagenesis and epigenesis) that overlapped the Azul sedimentary sequence belonging to the Águas Claras formation.
CONCLUSIONS
Azul’s manganese ore deposits are of Paleoproterozoic age and typically sedimentary-diagenetic associated with black shales hosted by reddish mudstones/siltstones and sandstones deposited in a shallow marine platform with expressive carbonaceous organic matter. Among CMP’s manganese deposits, it was the largest, with reserves in the process of being exhausted. It is dominated by Mn-oxyhydroxides, mainly cryptomelane and cryptomelane-hollandite, and its precursors are probably amorphous and/or low crystallinity Mn-oxyhydroxides. Mn-carbonates, for example, rhodochrosite, are rare and when present, of early diagenetic origin, and even epigenetic. The Mn-bearing sedimentary sequence is not metamorphized, with at most anchimetamorphic alteration suggested by the presence of sericite, sometimes chlorite, which may also be linked to diagenesis and tectonic deformations during two events. The source of Mn is mainly hydrogenous, also with a hydrothermal contribution demonstrated by the chemical composition, mainly by trace elements and REE and isotopic evaluations. The deposition and fixation of Mn from sea water was partly microbial mediated, for example, silicium bacteria, by oxidation, close to the redoxcline line, which locally gave rise to stromatolite-like structures. Pyrite framboids are present and organic in origin, with marine sulphate being reduced by bacterial action after isotopic data formed during the diagenesis.
With these characteristics, the Azul manganese deposits belong to deposits formed during the first Great Oxidation Event in the Earth’s atmosphere, between 2.4 and 2.1 Ga and are well correlated with several deposits from this period and in part with others from Neoproterozoic to Cenozoic.
Acknowledgements
The authors would like to thank ADIMB/FINEP for their financial support of this research; CNPQ for support through scholarships and research grants (303233/2003-4; 307901/2006-8; 304519/2009-0; 305015/2016-8); the ZWL in the name of Dr. Jürgen Göske and the University of Halle-Wittenberg for analytical support in the name of prof. Dr. Herbert Pöllmann; and the chemist Dr. Suyanne Flavia Santos Rodrigues and geologists Alessandro Sabá Leite and Daiveson Serrão for graphic support of the illustrations; and especially the two anonymous reviewers for the careful review of the manuscript as a whole.
REFERENCES
AKAI, J., AKIYAMA, S., TSUCHIYAMA, A., AKAI, K. 2013. Ocean manganese nodules as stromatolite with a fractal like-signature. Physics and Chemistry of the Earth 58–60 (2013) 42–48.
ANDERSON, W.L., DYER, R.C. & TORRES, D.D., 1974. Ocorrências de manganês na bacia do rio Itacaiúnas, Centro-Leste do estado do Pará. In: Congresso Brasileiro de Geologia, 28. Porto Alegre, Anais, p.149-164.
ANGERER, T., HAGEMANN, S.G., WALDE, D.H.G., HALVERSON, G.P., BOYCE, A.J. 2016. Multiple metal source in glaciomarine facies of the Neoproterozoic Jacadigo iron formation in the “Santa Cruz deposit”, Corumbá, Brazil. Precambr. Res. 275, 369–393.
ARAÚJO, O.J.B., JORGE-JOÃO X.S., COSTA J.B.S., 1988. A megaestruturação arqueana da Folha Serra do Carajás. In: SBG, Cong. Latino Amer. Geol., 7, Belém, Anais, 324-333.
ARAÚJO, R.N., SOUSA, M.J., 2018. Área de Relevante Interesse Mineral, Província Mineral de Carajás, PA: estratigrafia e análise do minério de Mn de Carajás, áreas Azul, Sereno, Buritirama e Antônio Vicente. CPRM, Report 16, Províncias Minerais do Brasil (Brazil Mineral Provinces). http://rigeo.cprm.gov.br/jspui/handle/doc/20421. Pdf 418 MB.
BAU, M., KOSCHINSKY, A., 2009. Oxidative scavenging of cerium on hydrous Fe oxide: evidence from the distribution of rare earth elements and yttrium between Fe oxides and Mn oxides in hydrogenetic ferromanganese crusts. Geochem. J. 43 (1), 37–47.
BAU, M., SCHMIDT, K., KOSCHINSKY, A., HEIN, J., KUHN, T., USUI, A., 2014. Discriminating between different genetic types of marine ferro-manganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 381, 1–9.
BEAUVAIS, A., 1984. Concentrations manganesifères lateritiques. Étude petroligique de deux gîtes sur le roches sedimentaires précambriennes.Gisements de Moanda (Gabon) et Azul (Brésil). Thése, Univ. Poitiers. 155p.
BEAUVAIS. A., MELFI. A., NAHON. D. & TRESCASES. J.J., 1987. Pétrologie du gisement latéritique manganésifere d’Azul (Bresil). Mineralium Deposita, 22: 124-134.
BERNARDELLI, A.L. & BEISIEGEL, V. R., 1978. Geologia Econômica da Jazida de Manganês do Azul. In: Congresso Brasileiro de Geologia, 30, Recife, 1978. Anais do …. Recife, SBG, 1978, v.4, p.1431-1444.
BERNARDELLI, A.L., 1982. Jazida de Manganês do Azul. In: Simpósio de Geologia da Amazônia, 1. Belém, 1982. Anexo dos Anais do … Belém, 1982, p. 47-60.
BIONDI, J.C. & LOPEZ, M., 2017. Urucum Neoproterozoic–Cambrian manganese deposits (MS, Brazil) Biogenic participation in the ore genesis, geology, geochemistry, and depositional environment. Ore Geology Reviews, 91 (2017) 335-386. doi:10.1016/j.oregeorev.2017.09.018.
BIONDI, J.C., POLGÁRI, M., GYOLLAI, I., FINTOR, K., KOVÁCS, I., FEKETE, J., MOJZSIS, J., 2020. Biogenesis of the Neoproterozoic kremydilite manganese ores from Urucum (Brazil) – A new manganese ore type. Precambrian Research, 340 (2020) 105624. doi:10.1016/j.precamres.2020.105624.
BONATTI, E., KRAEMER, T., RYDELL, H. 1972. Classification and genesis of submarine iron–manganese deposits of the ocean floor. In: Horn, D.R. (Ed.), Ferromanganese Deposits of the Ocean Floor. Aren House, Harriman, pp. 473–489.
BONHOMME. G.M., CORDANI. U.G., KAWASHITA. K., MACEDO. H.F., THOMAS FILHO. A., 1982. Radio chronological age and correlation of Proterozoic sediments in Brazil. Precambrian Research. 18(1/2): 103-118.
CABRAL, A.R., CREASER, R.A., NÄGLER, T., LEHMANNA, B., VOEGELIN, A.R., BELYATSKY, B., PAŠAVA, J., SEABRA GOMES JR., A.A., GALBIATTI, H., BÖTTCHER, M.E., ESCHER, P. 2013. Trace-element and multi-isotope geochemistry of Late-Archean black shales in the Carajás iron-ore district, Brazil. Chemical Geology 362 (2013): 91–104.
CABRAL, A.R., BÜH, B., GOMES, A.A.S., GALBIATTI, H.F., LEHMANN, B., HALDER, S. 2017. Multiple sulfur isotopes from the Neoarchaean Serra Sul black shale, Carajás mineral province, northern Brazil. Journal of South American Earth Sciences 79 (2017): 377-383. https://doi.org/10.1016/j.jsames.2017.08.002.
CHISONGA B.C. GUTZMER, J., BEUKES N.J., HUIZENGA, J.M. 2012. Nature and origin of the protolith succession to the Paleoproterozoic Serra do Navio manganese deposit, Amapa Province, Brazil. Ore Geology Reviews 47 (2012) 59–76.
COELHO, C.E.S & RODRIGUES, O.B., 1986. Jazida de Manganês do Azul, Serra dos Carajás, Pará. Principais depósitos Minerais do Brasil. DNPM/CVRD, v.2, p. 145-152.
CONLY, A.G., SCOTT, S.D. AND BELLON, H. 2011. Metalliferous Manganese Oxide Mineralization Associated with the Boléo Cu-Co-Zn District, Mexico. Economic Geology, 106: 1173–1196. Doi: 10.2113/econgeo.106.7.1173.
COSTA. M.L. CHOQUE FERNANDEZ. O. J. REQUELME. M.E.R., 2005. O depósito de manganês do Azul. Carajás: Estratigrafia. mineralogia. geoquímica e evolução geológica. In: O. J. MARINI et al. (Orgs.) – Caracterização de depósitos minerais em distritos mineiros da Amazônia. P. 231-333.
COSTA, M. L., 1997. Lateritisation As a Major Process of Ore Deposit Formation in the Amazon Region. Exploration and Mining Geology, 6: 79-104.
DARDENE, M.A. & SCHOBBENHAUS, C., 2001. Metalogênese do Brasil. Brasília: Editora Universidade de Brasília, 392p.
DENG, X-D., LI, J-W., VASCONCELOS, P.M., 2016. 40Ar/39Ar dating of supergene Mn-oxides from the Zunyi Mn deposit, Guizhou Plateau, SW China: Implications for chemical weathering and paleoclimatic evolution since the late Miocene. Chemical Geology, 445 (2016) 185–198. http://dx.doi.org/10.1016/j.chemgeo.2016.02.009.
DU, Q., YI, H, HUI, B., LI, S., XIA, G., YANG, W., WU, X. 2013. Recognition, genesis and evolution of manganese ore deposits in southeastern China. Ore Geology Reviews 55 (2013) 99–109. http://dx.doi.org/10.1016/j.oregeorev.2013.05.001.
DUBOIS, M., LOPEZ, M., OBERGER, B., GAY, A., MOUSSAVOU, M., PAMBO, F., RODRIGUES, S. 2017. 2.1Ga-old injectite network of the Franceville Basin, Gabon_ Architecture, origin and implications on manganese mineralization. Precambrian Research, 302 (2017) 255-278. doi:10.1016/j.precamres.2017.09.022.
FABRE, S., NÉDÉLEC, A., POITRASSON, F., STRAUSS, H., THOMAZO, C., NOGUEIRA, A., 2011. Iron and sulphur isotopes from the Carajás mining province (Pará, Brazil): implications for the oxidation of the ocean and atmosphere across the Archaean–Proterozoic transition. Chem. Geol. 289: 124–139. Doi:10.1016/j.chemgeo.2011.07.019.
FAN, D. & YANG, P. 1999. Introduction to and classification of manganese deposits of China. Ore Geology Reviews 15 (1999): 1–13.
FAN, D., YE, J., YIN, L., ZHANG, R.1999. Microbial processes in the formation of the Sinian Gaoyan manganese carbonate ore, Sichuan Province, China. Ore Geology Reviews 15 (1999): 79–93.
FRAKES. L & BOLTON. B., 1992. Effects of ocean chemistry. sea level. and climate on the formation of primary sedimentary manganese ore deposits. Economic geology 87 (5): 1207-1217.
GLASBY, G.P., PAPAVASSILIOU, C.T., MITSIS J., VALSAMI-JONES, E., LIAKOPOULOS, A., AND RENNER, R.M. 2005. The Vani manganese deposit, Milos Island, Greece: A fossil stratabound Mn-Ba-Pb-Zn-As-Sb-W-rich hydrothermal deposit. The South Aegean Active Volcanic Arc (M. Fytikas and G.E. Vougioukalakis), Elsevier, p. 25-291
GOLDHABER. M.B., 2004. Sulfur-rich sediments. In: F.T. Mackenzie. H.D. Holland and K.K. Turekian Treatise on geochemistry. Sediments. digenesis. and sedimentary rocks. Elsevier. Oxford. vol. 7: 257-288.
GOMES. C.B., CORDANI. U.G., BASEI. M.A.S., 1975. Radiometric ages from Serra dos Carajás. northern Brazil. Geol. Soc. Am. Bull., 86 (7): 939-945.
GHOSH, R., CHAKRABORTY, D., HALDER, M., BAIDYA, T.K. 2015. Manganese mineralization in Archean greenstone belt, Joda–Noamundi sector, Noamundi basin, East Indian Shield. Ore Geology Reviews 70 (2015): 96–109. http://dx.doi.org/10.1016/j.oregeorev.2015.04.007.
GUTZMER, J. & BEUKES, N.J. 1996. Mineral paragenesis of the Kalahari manganese field, South Africa. Ore Geology Reviews 11 (1996): 405-428.
GUTZMER, J., DU PLOOY, A.P., BEUKES, N.J. 2012. Timing of supergene enrichment of low-grade sedimentary manganese ores in the Kalahari Manganese Field, South Africa. Ore Geology Reviews 47 (2012) 136–153. doi: 10.1016/j.oregeorev.2012.04.003.
HARALYI, N.L.E. & WALDE, D.H.G., 1986. Os minérios de ferro e manganês da região de Urucum, Corumbá, Mato Grosso do Sul. In: Depósitos Minerais do Brasil, p. 127-144.
HEIN, J.R., KOSCHINSKY, A., HALBACH, P., MANHEIM, F.T., BAU, M., KANG, J.-K., LUBICK, N. 1997. Iron and manganese oxide mineralization in the Pacific. In: Nicholson, K., Hein, J.R., Buhn, B., Desgupta, S. (Eds.), Manganese Mineralization: Geochemistry and Mineralogy of Terrestrial and Marine Deposits. Geological Society Special Publication, 119: 123–138.
JONES, S., MCNAUGHTON, N.J., GRGURIC, B. 2013. Structural controls and timing of fault-hosted manganese at Woodie Woodie, East Pilbara, Western Australia. Ore Geology Reviews 50 (2013): 52–82.
JOHNSON, J.E., Webb, S.M., Ma, C., Fischer, W.W. 2016. Manganese mineralogy and diagenesis in the sedimentary rock record. Geochimica et Cosmochimica Acta 173 (2016): 210–231. http://dx.doi.org/10.1016/j.gca.2015.10.027.
KIMURA, H., MATSUMOTO. R., KAKUWA, Y., HAMDI, B., ZIBASERESHT, H. 1997. The Vendian-Cambrian S 13C record, North Iran: evidence for overturning of the ocean before the Cambrian Explosion. Earth and Planetary Science Letters 147 (1997) El-E7.
KLEIN, C., LADEIRA, E.A., 2004. Geochemistry and mineralogy of Neoproterozoic banded iron-formations and some selected, siliceous manganese formations form the Urucum district, Mato Grosso do Sul, Brazil. Econ. Geol. 99, 1233–1244.
KULESHOV, V.N. 2003. Isotopic Composition (δ13C, δ18O) and Origin of Manganese Carbonate Ores from the Early Oligocene Deposits, the Eastern Paratethys. Chem. Erde 63 (2003), 329–363. 10.1078/0009-2819-00029.
KULESHOV. V.N. 2017. Isotope geochemistry: the origin and formation of manganese rocks and ores. J.B. Maynard (ed.). Isotope Geochemistry. Elsevier. Amsterdam. 427p.
LAZNICKA. P., 1992. Manganese deposits in the global lithogenetic systems: a quantitative approach. Ore Geology Review. 7: 279-356.
LAN, Z., SANO, Y., YAHAGI, T., TANAKA, K., SHIRAI, K., PAPINEAU, D., SAWAKI, Y., OHNO, T., ABE, M., YANG, H., LIU, H., JIANG, T., WANG, T. 2019. An integrated chemostratigraphic (δ13C-δ18O-87Sr/86Sr-δ15N) study of the Doushantuo Formation in western Hubei Province, South China. Precambrian Research, 320 (2019): 232-25. 10.1016/j.precamres.2018.10.018.
LIAKOPOULOS, A.; GLASBY, G.P.; PAPAVASSILIOU, C.T. BOULEGUE, J., 2001. Nature and origin of the Vani manganese deposit, Milos, Greece: an overview. Ore Geology Reviews, 18:181-209.
MAYNARD. J.B., 2004. Manganiferous sediments. rocks and ores. In: F.T. Mackenzie. H.D. Holland and K.K. Turekian Treatise on geochemistry. Sediments. digenesis. and sedimentary rocks. Elsevier. Oxford. vol. 7: 289-308.
MOUGEOT, R., RESPAUT, J.P., BRIQUEU, L., LEDRU, P., MILESI, J.P., LEROUGE, C., MARCOUX, E., HUHN, S.B., MACAMBIRA, M.J.B., 1996. Isotope geochemistry constraints for Cu, Au mineralizations and evolution of the Carajás 763 province (Para, Brazil). 39e Congresso Brasileiro de Geologia, Salvador, Anais, SBG, vol 7: 321–324.
MOLNÁR, Z., POLGÁRI, M., HEIN, J.R., JÓZSA, S., FEKETE, J., GYOLLAI, I., FINTOR, K., BÍRÓ, L., SZABÓ, M., RAPI, S., FORGÓ, P., VIGHH, T. 2017. Fe-Mn oxide indications in the feeder and mound zone of the Jurassic Mn-carbonate ore deposit, Úrkút, Hungary. Ore Geology Reviews 86 (2017): 839–855.
NAYAK, B., DAS, S.K., MUNDA, P. 2013. Biogenic signature and ultra-microfossils in ferromanganese nodules of the Central Indian Ocean Basin. Journal of Asian Earth Sciences 73 (2013): 296–305. http://dx.doi.org/10.1016/j.jseaes.2013.03.032.
NOGUEIRA, A. C. R., TRUCKENBRODT, W., PINHEIRO, R. V. L.,1995. Formação Águas Claras, Pré-Cambriano da Serra dos Carajás: Redescrição e Redefinição Litoestratigráfica. Boletim do Museu Paraense Emilio Goeldi. Zoologia, Belém, v. 7: 177-197
PARK, J.H., KIM, B-S., CHON, C-M. 2018. Characterization of iron and manganese minerals and their associated microbiota in different mine sites to reveal the potential interactions of microbiota with mineral formation. Chemosphere 191 (2018): 245 – 252.
https://doi.org/10.1016/j.chemosphere.2017.10.050.
PERRY, R.S., MCLOUGHLIN, N., LYNNE, B.Y., SEPHTON, M.A., OLIVER, J.D., PERRY, C.C., CAMPBELL, K., ENGEL, M.H., FARMER, J.D., BRASIER, M.D., STALEY, J.T. 2007. Defining biominerals and organominerals: Direct and indirect indicators of life. Sedimentary Geology 201 (2007): 157–179. doi:10.1016/j.sedgeo.2007.05.014.
PERSEIL, E.A., 1998. Evolution cristallochimique des oxydes de manganèse dans le gisement de St. Marcel-Praborna (V. Aoste, Italie). Rend.Fis. Acc. Lincei., s. 9, v. 9, p.: 315-336.
PINHEIRO, R.V. AND HOLDSWORTH, R.E. 2000. Evolução tectonoestratigráfica dos sistemas transcorrentes Carajás e Cinzeiro, Cinturão Itacaiúnas, na borda leste do Cráton Amazônico, Pará. Brazilian Journal of Geology (Former Revista Brasileira de Geociências), 30(4):597-606. DOI: 10.25249/0375-7536.2000304597606.
POLGÁRI, M., HEIN, J.R., VIGH, T., SZABÓ-DRUBINA, M., FÓRIZS, I., BÍRÓ, L., MÜLLER, A., TÓTH, A.L., 2012. Microbial processes and the origin of the Úrkút manganese deposit, Hungary. Ore Geol. Rev. 47, 87–109.
POLGÁRI, M., HEIN. J.R., BÍRÓ, L., GYOLLAI, I., NÉMETH, T., SAJGÓ, C., FEKETE, J., SCHWARK, L., PÁL-MOLNÁR, E., HÁMOR-VIDÓ, M., VIGH, T. 2016. Mineral and chemostratigraphy of a Toarcian black shale hosting Mn-carbonate microbialites (Úrkút, Hungary). Palaeogeography, Palaeoclimatology, Palaeoecology 459 (2016) 99–120.
PUTTER, T., RUFFET, G., YANS, J., MEES, F. 2015. The age of supergene manganese deposits in Katanga and its implications for the Neogene evolution of the African Great Lakes Region. Ore Geology Reviews 71 (2015) 350–362. http://dx.doi.org/10.1016/j.oregeorev.2015.06.015.
PUTTER, T., LIÉGEOIS, J-P., DEWAELE, S., CAILTEUX, J., BOYCE, A., MEES, F. 2018. Paleoproterozoic manganese and base metals deposits at Kisenge-Kamata (Katanga, D.R. Congo). Ore Geology Reviews 96 (2018) 181–200. https://doi.org/10.1016/j.oregeorev.2018.04.015.
RAJABZADEH, M.A., HADDAD, F., POLGÁRI, M., FINTOR, K., WALTER, H., MOLNÁR, Z., GYOLLAI, I. 2017. Investigation on the role of microorganisms in manganese mineralization from Abadeh-Tashk area, Fars Province, southwestern Iran by using petrographic and geochemical data. Ore Geology Reviews 80 (2017): 229–249.
REOLID, M., EL KADIRI, K., ABAD, I., OLÓRIZ, F., JIMÉNEZ-MILLÁN, J. 2011. Jurassic microbial communities in hydrothermal manganese crust of the Rifian Calcareous Chain, Northern Morocco. Sedimentary Geology 233 (2011) 159–172. doi: 10.1016/j.sedgeo.2010.11.008.
ROY, S. BANDOPADHYAY, P.C., PERSEIL, E.A., FUKUOKA, M.,1990. Late Diagenetic Changes in Manganese Ores of the Upper Proterozoic Penganga Group, India. Ore Geology Reviews, 5 (1990) 341-357.
ROY. S., 2006. Sedimentary manganese metallogenesis in response to evolution Earth System. Journal Earth Sciences Reviews. 77: 273-305.
RUFFET. G., INNOCENT. C., MICHARD. A., FÉRAUD.G., BEAUVAIS.A., NAHON, D. & HAMELIN, B., 1996. A geochronological 40Ar/39Ar and 87Rb/87Sr study of K-Mn oxides from the weathering sequence of Azul. Brazil. Geochimica et Cosmochimica Acta. 60 (12): 2219-2232.
SACKETT, W.M.1986. ð13C signatures of organic carbon in southern high latitude deep sea sediments; paleotemperature implications. Org. Geochem, 9 (2): 63-68.
SAGEMAN, B.B., 2004. Geochemistry of fine-grained sediments and sedimentary rocks. In: F.T. Mackenzie. H.D. Holland and K.K. Turekian Treatise on geochemistry. Sediments. digenesis. and sedimentary rocks. Elsevier. Oxford. vol. 7: 115-158.
SALGADO. S. S., CAXITO. F.A., QUEIROGA. G.N., CASTRO. M.P., 2019. Stratigraphy, petrography and tectonics of the manganese-bearing of the Buritirama Formation. Northern of Carajás Domain. Amazon Craton. Brazilian Journal of Geology. 49(1): 1-15. Doi: 10.1590/2317-4889201920180106.
SCARPELLI, W. AND HORIKAVA, E.H., 2017. Gold, iron and manganese in central Amapá, Brazil. Brazilian Journal of Geology, 47(4): 703-721.
SCHRÖDER, S., BEKKER, A., BEUKES, N.J., STRAUSS, H., VAN NIEKERK, H.S. 2008. Rise in seawater sulphate concentration associated with the Paleoproterozoic positive carbon isotope excursion: evidence from sulphate evaporites in the ∼2.2–2.1 Gyr shallow‐marine Lucknow Formation, South Africa. Terra Nova, Vol20, No. 2, 108–117. doi: 10.1111/j.1365-3121.2008.00795.x
SCOTT, C., WING, B.A., BEKKER, A., PLANAVSKY, N.J., MEDVEDEV, P., BATES, S.M., YUN, M., LYONS, T.W. 2014. Pyrite multiple-sulfur isotope evidence for rapid expansion and contraction of the early Paleoproterozoic seawater sulfate reservoir. Earth and Planetary Science Letters, 389 (2014): 95-104. https://doi.org/10.1016/j.epsl.2013.12.010
SETHUMADHAV, M.S., GUNNELL, Y., AHMED, M.M., CHINNAIAH. 2010. Late Archean manganese mineralization and younger supergene manganese ores in the Anmod–Bisgod region, Western Dharwar Craton, southern India: Geological characterization, palaeoenvironmental history, and geomorphological setting. Ore Geology Reviews 38 (2010): 70–89.
SHIRAISHI, F., MITSUNOBU, S., SUZUKI, K., HOSHINO, T., MORONO, Y., INAGAKI, F. 2016. Dense microbial community on a ferromanganese nodule from the ultra-oligotrophic South Pacific Gyre: Implications for biogeochemical cycles. Earth and Planetary Science Letters, 447(2016): 10–20. http://dx.doi.org/10.1016/j.epsl.2016.04.021
SILVA, J.L. DA., 1988. Mina de manganês do Azul. In: Congresso Brasileiro de Geologia, 35, Belém, 1988. Província Mineral do Carajás, Litoestratigrafia e principais depósitos minerais; Anexo dos Anais, Belém, p.73-94.
SINISI, R., MONGELLI, G., PERRI, F., RIZZO, G. 2018. The braunite (3Mn2O3·MnSiO3)-rich mineralization in the metasedimentary succession from southern Apennines (Italy): Genesis constraints. Ore Geology Reviews 94 (2018) 1–11.
TANG. S. AND LIU. T., 1999. Origin of the early Sinian Minle manganese deposit. Hunan Province. China. Ore Geology Reviews 15. 71–78.
TAYLOR, S.R. AND MCLENNAN, S.M., 1985. The Continental Crust: Its Composition and Evolution. Blackwell, Oxford, 1-312.
VAFEAS, N.A., VILJOEN, K.S., BLIGNAUT, L.C. 2018. Mineralogical characterization of the thrusted manganese ore above the Blackridge Thrust Fault, Kalahari Manganese Field: The footprint of the Mukulu Enrichment. Island Arc. 2019; 28:e12280. wileyonlinelibrary.com/journal/iar © 2018 John Wiley & Sons Australia, Ltd 1 of 13. https://doi.org/10.1111/iar.12280.
VALARELLI, J.V., BERNARDELLI, A.L. & BEISIEGEL, V.R., 1978. Aspectos Genéticos do Minério de Manganês do Azul. In: Congresso Brasileiro de Geologia, 30, Recife, 1978. Anais. Recife, SBG, 1978, v.4, p.1670-1979.
VALARELLI. J., COUTINHO. J.M.V., BELLO. R.M.S., 1978. Metamorfismo de Buritirama. Pará. In: Congresso Brasileiro de Geologia. 30.. Recife. Proceedings… 3:1357-1363.
VALARELLI. J.V., BERNARDELLI. A.L. & BEISIEGEL.V.R., 1978. Aspectos Genéticos do Minério de Manganês do Azul. In: Congresso Brasileiro de Geologia. 30. Recife. 1978. Anais. Recife. SBG. 1978. v.4. p.1670-1979.
VARENTSOV, I.M. 2002. Genesis of the Eastern Paratethys manganese ore giants: impact of events at the Eocene/Oligocene boundary. Ore Geology Reviews 20 (2002) 65.
VASCONCELOS, P.M., RENNE, P.R., BRIMHALL, G.H. & BECKER, T.A., 1994. Direct dating of weathering phenomena by 40Ar/39Ar and K-Ar analysis of supergene K-Mn oxides. Geochimica et Cosmochimica Acta, 58 (6), 1635-1665.
VASCONCELOS, P.M., RENNE, P.R., BRIMHALL, G.H. & BECKER, T.A., 1994. Direct dating of weathering phenomena by 40Ar/39Ar and K-Ar analysis of supergene K-Mn oxides. Geochimica et Cosmochimica Acta. 58 (6). 1635-1665.
VASQUEZ, M. L., ROSA-COSTA, L.T., SILVA, C.M.G., KLEIN, E.L., 2008. Compartimentação Tectônica. In: Vasquez, M. L., Rosa-Costa, L.T. 2008. (eds.). Geologia e recursos minerais do estado do Pará: Sistema de Informações Geográficas – SIG: texto explicativo dos mapas geológico e tectônico e de recursos minerais do estado do Pará. Belém, PA, CPRM, 1 CD-ROM.
VIEHMANN, S., BAU, M., BÜHN, B., DANTAS, E.L., ANDRADE, F.R.D., WALDE, D.H.2016. Geochemical characterization of Neoproterozoic marine habitats: Evidence from trace elements and Nd isotopes in the Urucum iron and manganese formations, Brazil. Precambrian Research 282 (2016): 74–96. http://dx.doi.org/10.1016/j.precamres.2016.07.006.
XIAO, J.F., HE, J.Y., YANG, H.Y., WUA, C. 2017. Comparison between Datangpo-type manganese ores and modern marine ferromanganese oxyhydroxide precipitates based on rare earth elements. Ore Geology Reviews 89 (2017): 290–308.
YU, W., ALGEO, T.J., DU, Y., MAYNARD, B., GUO, H., ZHOUD, Q. PENG, T., WANGA, P., YUAN, L. 2016. Genesis of Cryogenian Datangpo manganese deposit: Hydrothermal influence and episodic post-glacial ventilation of Nanhua Basin, South China. Palaeogeography, Palaeoclimatology, Palaeoecology 459 (2016) 321–337.
YU, W., ALGEO, T.J., DU, Y., ZHOU, Q., WANG, P., XU, Y., YUAN, L., PAN, W., 2017. Newly discovered Sturtian cap carbonate in the Nanhua Basin, South China. Precambr. Res. 293, 112–130.
YU, W., POLGÁRI, M., GYOLLAI, I., FINTOR, K., SZABÓ, M., KOVÁCS, I., FEKETE, J., DU, Y., ZHOUE, Q. 2019. Microbial metallogenesis of Cryogenian manganese ore deposits in South. China. Precambrian Research 322 (2019): 122–135.
WEBER. F., 1997. Evolution of lateritic manganese deposits. In: Paquet H., Clauer, N. Soils and sediments-mineralogy and geochemistry. Springer-Verlag. eds.pp.: 97-124.
ZARASVANDI, A., LENTZ, D., REZAEI, M., POURKASE, H. 2013. Genesis of the Nasirabad manganese occurrence, Fars province, Iran: Geochemical evidences. Chemie der Erde 73 (2013): 495 – 508. http://dx.doi.org/10.1016/j.chemer.2013.02.003.
ZEINER. C.A., LION. L.W., SHULE, M.E., GHIROSE, W.C., 2006. Cycling of biogenic Mn-oxides in the model of microbial predator-prey system. Geomicrobiology Journal. 23(1): 37-43.
http://www.webmineral.com/data/Clinochlore.shtml#.XnjQBIhKjIU, accessed March 23, 2020.