01 – TRANSFORMATION OF BAUXITE TAILINGS INTO SR-CANCRINITE
Ano 12 (2025) – Número 3 – Mineral Synthesis Artigos

10.31419/ISSN.2594-942X.v122025i3a1NAFC
Nayara Aparecida Fonseca Couto1, Igor Alexandre Rocha Barreto2, Magno M. da Luz Sousa3, Andson Pereira Ferreira4, Bruno Apolo Miranda Figueira1*
1Instituto Federal do Pará, IFPA-Campus Belém, Programa de Pós-Graduação em Engenharia de Materiais – PPGEMAT, Belém, Pará, Brasil. figueiraufpa@gmail.com
2Universidade Federal do Pará, UFPA-Campus Belém, Instituto de Geociências – IG, Belém, Pará, Brasil.
3Faculdade de Química, UFPA-Campus Ananindeua, Pará, Brasil.
4Instituto Federal do Pará, IFPA-Campus Parauapebas, Pará, Brasil.
* Author for correspondences
Received in June 2024; revised and accepted on December 15, 2024.
ABSTRACT
This research investigated the feasibility of obtaining a zeolitic material with a Sr-cancrinite structure using gibbsite bauxite tailings (BLT) from the Amazon. The synthetic route employed consisted of 3 stages: i) acid leaching of BTL to remove Fe oxide; ii) alkaline fusion of clay with NaOH reagent followed by hydrothermal treatment; iii) cation exchange of Na+ ions with Sr2+. The chemical-mineral characterization of the products was carried out by X-ray fluorescence, X-ray diffractometry, Fourier transform infrared spectroscopy and scanning electron microscopy. The results showed that the developed route was effective for the synthesis of Sr-cancrinite, which showed well-defined needle morphology, thermal stability at high temperatures and phase transition to SrAl2Si2O8.
Keywords: bauxite tailings, conversion, Sr-cancrinite, SrAl2Si2O8.
INTRODUCTION
Mining is the fundamental basis of the Brazilian economy, occupying a prominent position among the country’s productive sectors (IBRAM, 2023). Favored by its rich geodiversity, Brazil has one of the greatest mineral potentials in the world (Cordani and Caetano, 2019). The Northern region stands out for being home to extensive deposits of strategic minerals, such as bauxite, iron, manganese, copper, nickel, gold and kaolin, which contribute significantly to socio-economic development (Martins et al., 2022).
Bauxite ores from the Amazon is considered to be of great economic value due to its chemical-mineral composition formed by gibbsite (Al(OH)3), which improves the production of alumina by the Bayer process. Other minerals are also present in the ore (mainly kaolinite and hematite) and are removed by the washing process which produces a by-product known as bauxite washing clay (Melo et al., 2019, Angélica et al., 2018; Reis et al., 2023).
Despite being classified as waste, bauxite washing clay shows promising potential as a low-cost source of Al and Si to produce technologically important materials such as zeolites-type materials, SBA-15 and MCM-41 (Melo et al., 2019; Mourão et al., 2023). The valorization of these by-products contributes to the sustainability of mining and opens up new opportunities for the development of materials with unique properties.
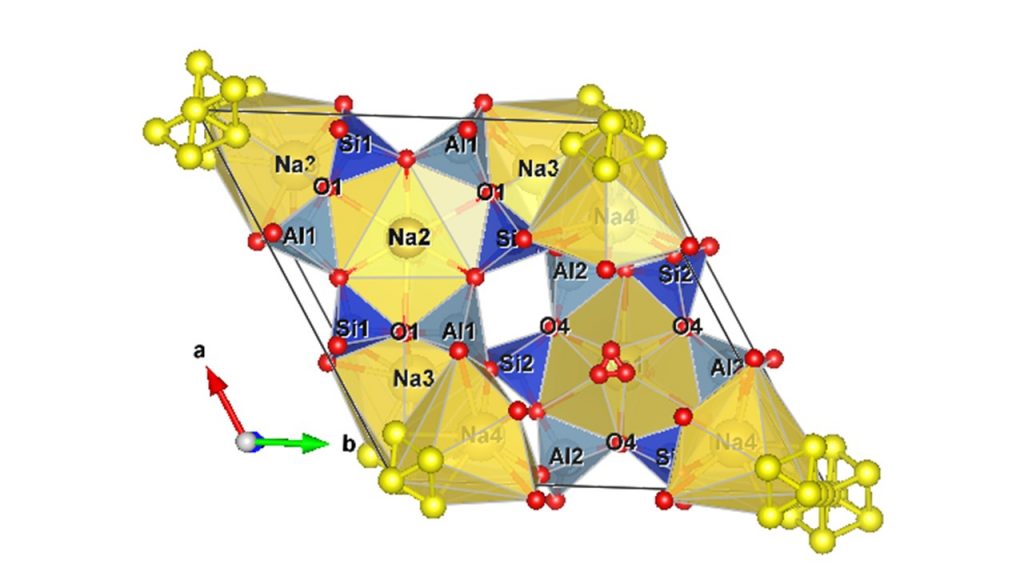
Zeolites comprise an extensive group of minerals from the tectosilicate group characterized by their microporous structure with different tunnel openings depending on the mineral (sodalite, faujasite, analcime, chabazite, mordenite, clinoptilolite, phillipsite, cancrinite, etc.). Their structure is made up of interconnected SiO4 and AlO4 tetrahedra with the presence of exchangeable mono- and divalent cations in the framework (Sels and Kustov, 2016). For cancrinite-type zeolite and its synthetic analog (Na8(Al6Si6O24)(OH)2(H2O)2.66), its structure is made up of Al and Si tetrahedrons with Na+, OH– ions (Fig. 1), in which Na+ cations can be easily exchanged for other cations from the alkali and alkaline earth metal family. Cancrinite displays channels made up of 12-membered rings, which are ~ 6.2 Å in size, giving it a high ion exchange capacity. When Na+ and OH– ions are exchanged for Ca2+, CO32- and SO42- ions, respectively, for example, other minerals can be formed, such as hydroxycancrinite and vishnevite (Gatta and Lotti, 2016, Teng et al., 2022).
Figure 1 – Illustration of the cancrinite structure obtained by Vesta® software.
In this study, bauxite washing clay from Amazon Region was investigated in order to evaluate its transformation into a value-added product, more specifically, strontium-cancrinite-type material through alkaline fusion method followed by hydrothermal treatment at low temperature.
MATERIALS AND METHODS
The starting material and commercial reagents used in this work were: bauxite tailings (BTL), sodium hydroxide (NaOH) (VETEC) and strontium chloride (SrCl2) (SYNTH). The route synthesis carried out in this work comprises 3 stages. Stage 1: acid leaching treatment of BTL for 4 h at 100º C. After the acid treatment, the suspension was filtered, washed and coded as BTL-LIX. Stage 2: A fused material was obtained by mixing BTL and NaOH in a 1:2 ratio, followed by hydrothermal treatment for 5 days at 90º C. The product obtained was washed with deionized water, dried and coded as Nay-CAN. Stage 3: it consisted of the ion exchange procedure of cancrinite-type material with Sr2+ using a 1 mol.L-1 SrCl2 solution for 24 h at room temperature. The material was washed, dried and coded as Nay-CAN-Sr.
The chemical composition of BTL was determined by X-ray fluorescence (Axios Minerals, from Panalytical) spectrometer. An X-ray diffractometer (XRD) (D2 PHASER, Bruker®) was used to perform the mineral characterization of the products under the following test conditions: Cu targeted Kα-Ray, 1.5406 Å wavelength, 30 kV working voltage, 10 mA tube current, 0.02 s step length, and 5–60° scanning range. The FT-IR analysis was performed on a Vertex 70 (Bruker®) spectrophotometer using the KBr pellet technique, in the frequency interval 4000-400 cm-1. The surface morphology of the synthetic products was made employing scanning electron microscopy (SEM, TESCAN Vega).
RESULTS AND DISCUSSIONS
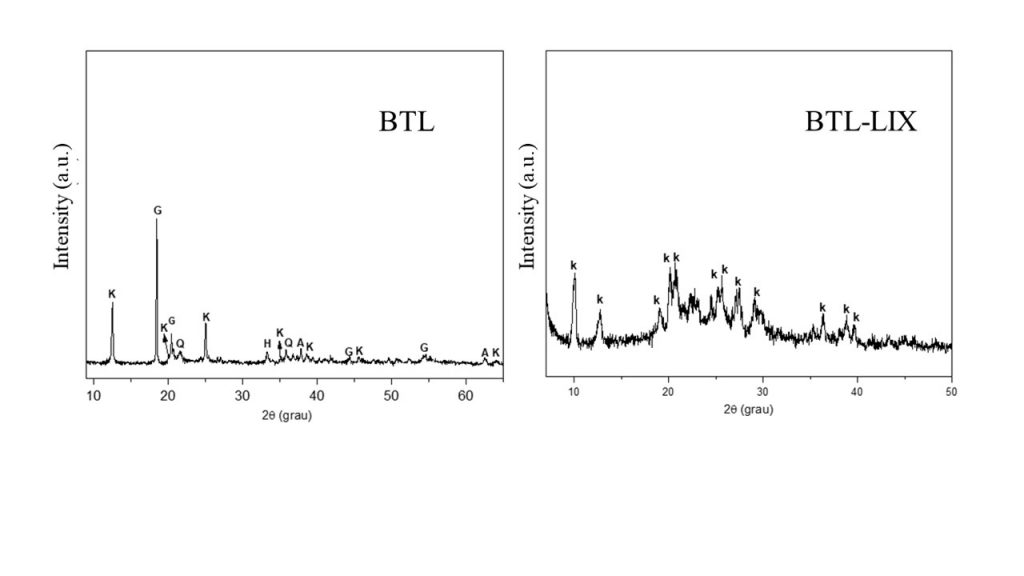
The chemical composition of BTL consisted of SiO2 (29.2 % wt %), Al2O3 (43.6 wt %), Fe2O3 (22.3 wt %) and TiO2 (3.17 %). The Si/Al molar ratio was 1.12. Based on the composition of cancrinite formed by Si and Al and Si/Al ratio of 1:1 (Reyes, Williams and Alarcón, 2013), the BTL sample was used as raw material for its synthesis. Fig 2 shows the XRD patterns of BTL and BTL-X. The main constituents of the BTL sample were monoclinic kaolinite-1 Md (PDF 00-003-0052), gibbsite (PDF 00-074-1775), hematite (PDF 01-084-0307), quartz (PDF 01-080-2147), and anatase ((PDF 01-078-2486), while only triclinic kaolinite 1A (PDF 01-074-1786) and quartz were present in BTL-LIX. These results indicated that the acid leaching process favored a change in the crystalline system of kaolinite (from monoclinic to triclinic), in addition to possibly removing Fe, Al, and Si oxides previously present in BTL.
Table 1 – Chemical composition of BTL sample by XRF. Table 1 –
|
Components |
Al2O3 | SiO2 | Fe2O3 | TiO2 | Total |
| w.t (%) | 43,6 | 29,2 | 22,3 | 3,17 | 99,6 |
Figure 2 – XRD patterns of raw material (BTL) and after acid leaching treatment (BTL-LIX). Legend = K – kaolinite, G – gibbsite, H – hematite, Q – quartz, A – anatase.
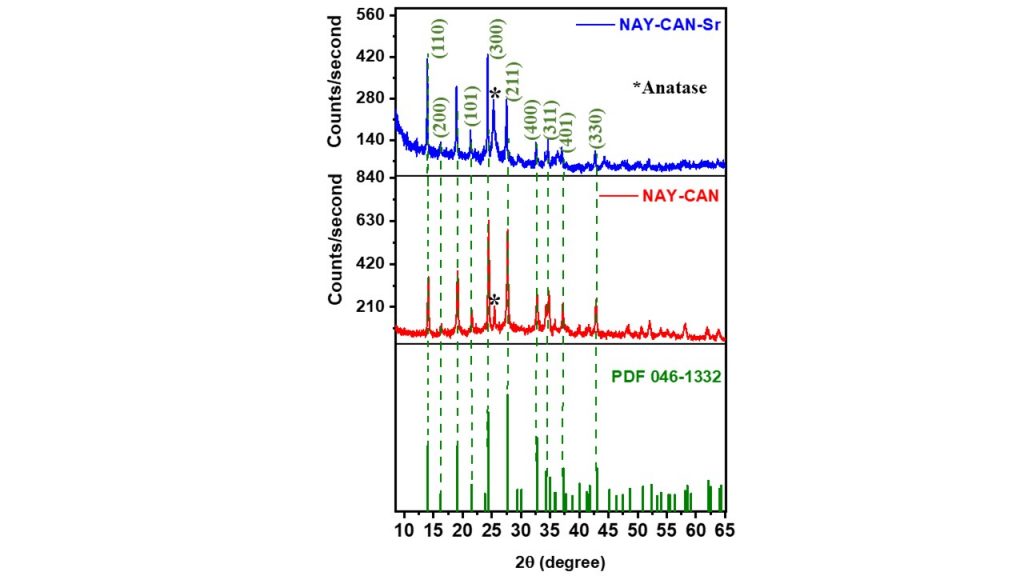
XRD patterns of the synthesized Na-cancrinite (NaY-CAN) and its strontium form (NaY-CAN-Sr) are showed in Fig. 3. The characteristic XRD peaks of cancrinite appeared clearly for both samples, although they decreased in intensity in the Nay-CAN-Sr sample, suggesting that the cation exchange in the cancrinite framework may have caused a structural disorder. Moreover, it was possible to measure the average crystallite size (ACS) by Scherrer equation and degree crystallinity (DC) of the Na-cancrinite (NAY-CAN) and Sr-cancrinite (NAY-Can-Sr) structures according to the Eq. 1 and Eq. 2, respectively. The results indicated that Na-cancrinite showed an average crystallite size of 27.5 nm and crystallinity of around 54 %, while Sr-cancrinite showed a crystallinity of 50 % and crystallite size of 124 nm, thus suggesting that the insertion of Sr2+ into the structure has not affected the degree of crystallinity but influenced the increase in crystal grain size.
ACS = K.l (Eq. 1)
b.cos q
DC (%) = Area crystalline peaks x 100 (Eq. 2)
Area of all peaks (crystalline + amorphous)
Figure 3 – XRD patterns of NAY-CAN and NAY-CAN-Sr samples.
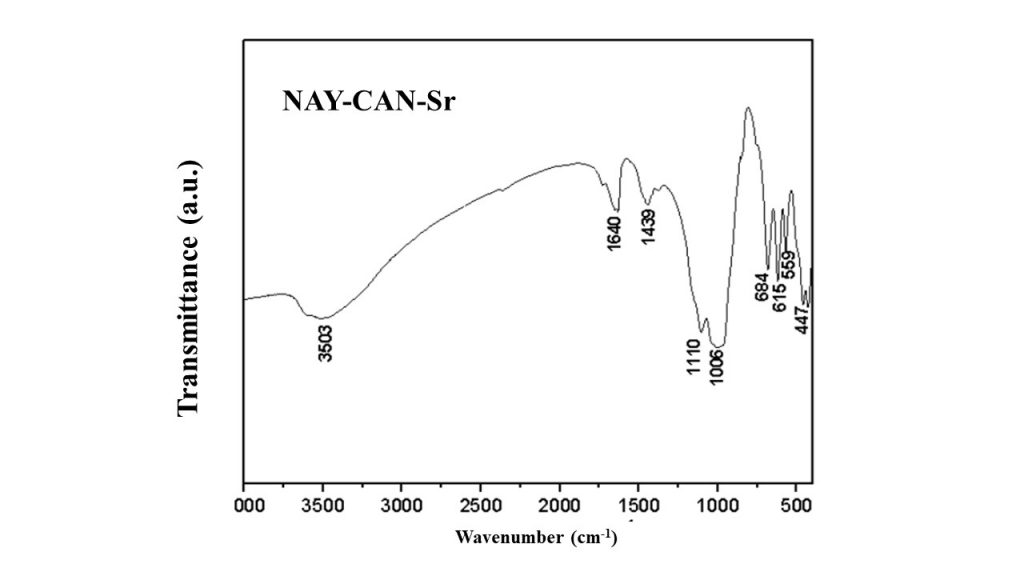
Figure 4 shows the FTIR spectra of the Sr-cancrinite (NAY-CAN-Sr) in the 4000 to 500 cm-1 range. According to Burton et al. (1999), the bands at 3600 and 1639 cm-1 correspond to water molecules located in the cancrinite cavities (BURTON, 1999). Bands related to the stretching of the Al-Si-O bonds of the TO4 tetrahedra (T = Si and Al) located in the cancrinite framework were also identified in the 800-400 cm-1 range. According to Ocanto et al. (2005) and Barnes et al. (1999), they are diagnostic of cancrinite and were identified at 559, 615 and 684 cm-1. As can be observed, the result of XRD analysis was confirmed by spectroscopic characterization: Sr-cancrinite was successfully synthesized by the proposed route, and the Fe, Al and Si oxides previously found in the tailings were clearly absent (or in very small quantities) in the final product.
Figure 4 – FTIR spectra of Sr-cancrinite (NAY-CAN-Sr).
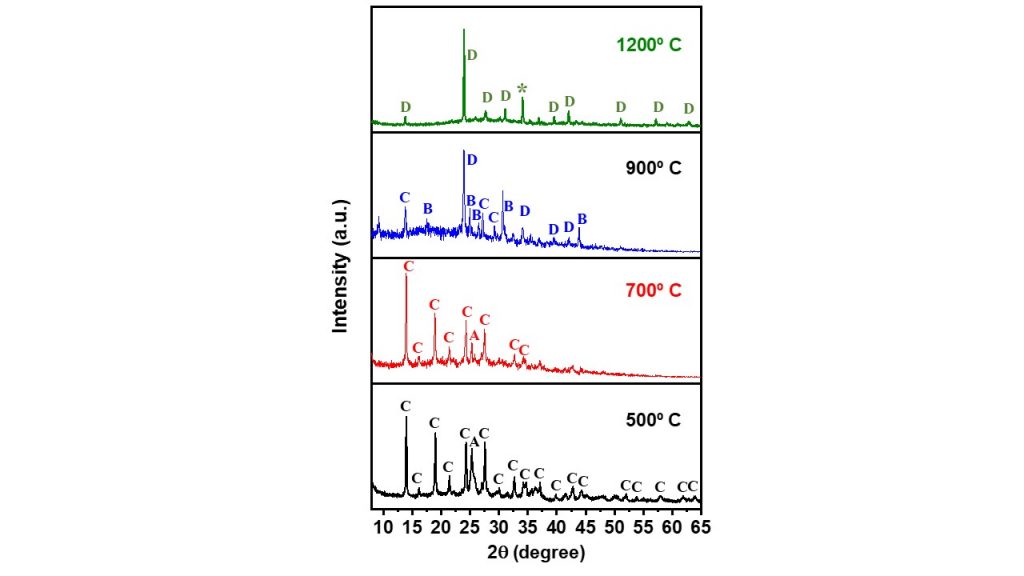
The XRD patterns of the NAY-CAN-Sr heated at different temperatures are shown in Fig. 5. In the temperature range of 500-700º C, the Sr-cancrinite phase remained stable, as identified by the XRD patterns, which also indicated a reduction in crystallite size from 27 nm to 20 nm, respectively. With increasing temperature, a reduction in crystallinity from 33 % to 20 % was also observed. Up to 900º C, trace phases like Sr-cancinite and SrSiO3 (PDF 036-0018) were observed, as well as a predominant phase of SrAl2Si2O8 (PDF 038-1454). A further increase in temperature (~1200º C) was accompanied by the complete formation of SrAl2Si2O8, and showed the occurrence of the Sr-cancrinite-SrAl2Si2O8 phase conversion at high temperature and a relatively short time (~ 60 min).
Figure 5 – XRD patterns of NAY-CAN-Sr heated at 500, 700, 900 and 1200º C. Legend = A – anatase, B – SrSiO3, C – Sr-crancrinite, D – SrAl2Si2O8, * – unknown phase.
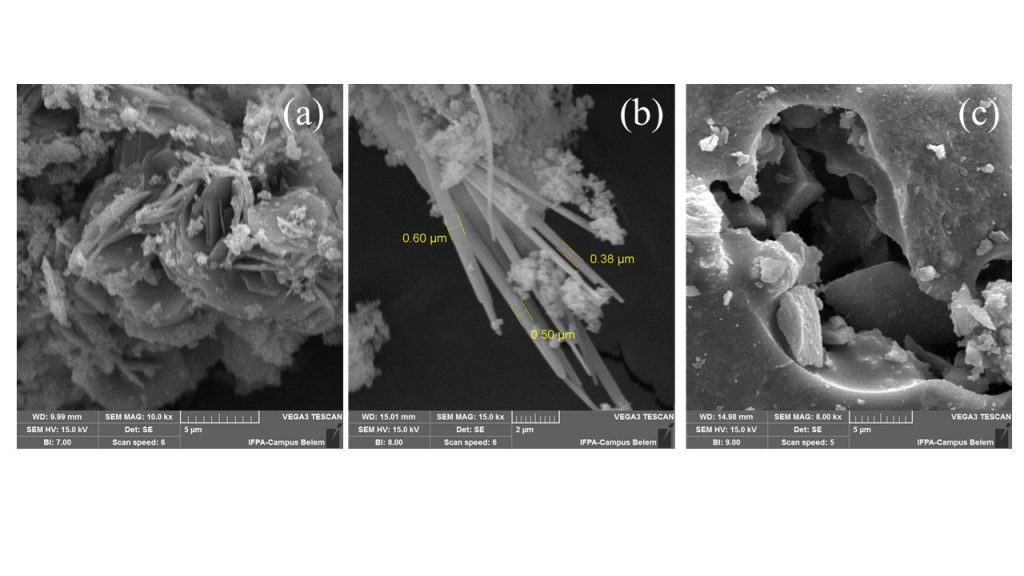
SEM images of the raw material after acid leaching (BTL-LIX), Na-cancrinite (NAY-CAN) and SrAl2Si2O8 (NAY-CAN-1200º C) are displayed in Fig. 6. Regarding to BTL-LIX (Fig. 6a), an agglomeration of plate particles around 5 mm length confirmed the XRD kaolinite 1A polytype, which is present in triclinic crystal system (Ece and Ercan, 2024). On the other hand, the SEM images in Figure 6b revealed that sodium cancrinite is in the form of stacks consisting of well-defined needle crystallites with an average thickness of 500 nanometers (0.50 mm) immersed in a base of undefined agglomerates, probably related to the amorphous phase as previously described in the X-ray diffractometry results. A porous structure morphology could be observed for the strontium aluminosilicate sample obtained above 1000º C (Fig. 6c).
Figure 6 – SEM photomicrographs of BTL-LIX (a), NAY-CAN-Sr (b) and NAY-CAN-Sr-1200º C (c).
CONCLUSIONS
The Sr-cancrinite-type material was synthesized employing bauxite washing clay from Amazonia, and its mineralogical properties investigated. The following are key results:
- a) The bauxite tailings were characterized by the presence of the minerals kaolinite, gibbsite, anatase, and hematite, as well as high contents of Al2O3, SiO2, and Fe2O3. After treatment by acid leaching of the tailings, they were converted into a suitable starting material for the hydrothermal synthesis of sodium cancrinite.
- b) The sodium cancrinite-type material was synthesized through a two-phase method, which, after cation exchange with SrCl2 solution was converted into Sr-cancrinite.
- c) Sr-cancrinite exhibited a high degree of crystallinity, needle-like morphology, high thermal stability (>750°C), and a phase transition to an important ceramic material based on strontium aluminosilicate, SrAl2Si2O8.
REFERENCES
Angélica, R. S.; Kahn, H.; Paz, S. P. A. 2018. A proposal for bauxite quality control using the combined Rietveld – Le Bail – Internal Standard PXRD method – Part 2: Application to a gibbsitic bauxite from the Paragominas region, northern Brazil. Minerals Engineering. v. 122, pp. 148–155. DOI: 10.1016/j.mineng.2018.03.039.
BARNES, M. C.; ADDAI-MENSAH, J.; GERSON, A. R. A. 1999. A methodology for quantifying sodalite and cancrinite phase mixtures and the kinetics of the sodalite to cancrinite phase transformation. Microporous and Mesoporous Materials, v. 31, pp. 303-319. https://doi.org/10.1016/S1387-1811(99)00080-3.
BURTON, A.; FEUERSTEIN, M.; Lobo, R. F.; CHAN, J. C. 1999. Characterization of cancrinite synthesized in 1, 3-butanediol by Rietveld analysis of powder neutron diffraction data and solid-state 23Na NMR spectroscopy. Microporous and mesoporous materials, v. 30, p. 293-305, 1999. https://doi.org/10.1016/S1387-1811(99)00040-2
CORDANI, U. G.; CAETANO, J. 2019. Potencial mineral da Amazônia: problemas e desafios. Revista de Estudios Brasileños, v. 6, pp. 91–108. DOI:10.14201/reb201961191108.
ECE, Ö.I.; ERCAN, H.Ü. 2024. Global Occurrence, Geology and Characteristics of Hydrothermal-Origin Kaolin Deposits. Minerals, v. 14, 353-401. https://doi.org/10.3390/min14040353
Gatta, G. D.; Lotti, P. 2016. Cancrinite-group minerals: Crystal-chemical description and properties under non-ambient conditions-A review. American mineralogist, v. 101, pp. 253–265. https://doi.org/10.2138/am-2016-5282.
IBRAM. 2023. Brazil Mining Overview. Brazilian Mining Association < https://panoramamineracao.com.br/pmb2024//> accessed December 14, 2024.
MARTINS, W. B. R.; RODRIGUES, J. I. DE M.; DE OLIVEIRA, V. P.; RIBEIRO, S. S.; BARROS, W. DOS S.; SCHWARTZ, G. 2022. Mining in the Amazon: Importance, impacts, and challenges to restore degraded ecosystems. Are we on the right way? Ecological Engineering, v. 174, p. 106468. DOI: 10.1016/j.ecoleng.2021.106468.
MELO, C.C.A.; MELO B.L.S; ANGÉLICA, R.S.; PAZ, S.P.A. 2019. Gibbsite-kaolinite waste from bauxite beneficiation to obtain FAU zeolite: Synthesis optimization using a factorial design of experiments and response surface methodology. Applied Clay Science, pp 125-134. DOI: 10.1016/j.clay.2019.01.010.
Mourão, L. V. O.; Rodrigues, A. O.; Angélica, R.S.; Paz, S.P.A. 2023. Otimização da síntese de analcima ‘condicionador de solo’ a partir de rejeito gibbsítico-caulinítico derivado de bauxita: uma análise de drx-cluster associada a uma matriz doe. Boletim do museu de geociências da Amazônia (BOMGEAM). Volume 10 (2023), p. 1-18. DOI: 10.31419/ISSN.2594-942X.v102023i1agromineraisa2LVOM
OCANTO, F.; LINARES, C. F., FIGUEIREDO, E.; NAVARRO, C. U. 2019. Antibacterial property of cancrinite-type zeolites exchanged with silver and copper cations. Revista Técnica de la Facultad de Ingeniería, Universidad del Zulia, v. 42, p. 143-151. https://www.redalyc.org/journal/6057/605766524006/html/
REIS, A.S., DUARTE, G.M.P., NEVES, E., OLIVEIRA, G., JATOBÁ, T. 2023. Process Simulation with Tertiary Cyclone for Kaolinite Removal from Amazonian Bauxite. The Minerals, Metals & Materials Series, Springer, pp. 147. https://link.springer.com/book/10.1007/978-3-031-22532-1.
REYES, C. A. R; Williams, C.; Alarcón, O. M. C. 2013. Nucleation and Growth Process of Sodalite and Cancrinite from Kaolinite-rich Clay under Low-temperature Hydrothermal Conditions. Materials Research. v. 16, pp. 424-438. https://doi.org/10.1590/S1516-14392013005000010.
SELS, B. F.; KUSTOV, L. M. 2016. Zeolites and Zeolite-Like Materials. Elsevier, pp. 459. https://doi.org/10.1016/C2014-0-00257-2.
TENG, L..; JIN, X.; BU, Y.; MA, J.; LIU Q.; YANG, J.; LIU, W.; YAO, L. 2022. Facile and fast synthesis of cancrinite-type zeolite from coal fly ash by a novel hot stuffy route. Journal of Environmental Chemical Engineering, v. 10, p. 108369. DOI: 10.1016/j.jece.2022.108369.