08 – MINERALOGICAL AND CHEMICAL DATA ON THE GEOSCIENCE MUSEUM COLLECTION SAMPLE 2478: Zn-Pb ORE
Ano 06 (2019) – Número 02 Artigos
![]() 10.31419/ISSN.2594-942X.v62019i2a8MLC
10.31419/ISSN.2594-942X.v62019i2a8MLC
Dados mineralógicos e químicos sobre a amostra 2478 de minério de Zn-Pb do Museu de Geociências
Marcondes Lima da Costa1
Glayce Jholy Souza da Silva Valente2
Pabllo Henrique Costa dos Santos3
1 Instituto de Geociências da Universidade Federal do Pará, Pesquisador do CNPq e Membro Titular da ABC e curador do Museu de Geociências, marcondeslc@gmail.com
2 PNPD-PPGG, Instituto de Geociências da Universidade Federal do Pará, glaycej@yahoo.com.br;
3 Doutorando do PPGG e técnico do Museu de Geociências, Instituto de Geociências da Universidade Federal do Pará, phsantosgeo@gmail.com
ABSTRACT
The beautiful sample 2478 recently incorporated into the collection of the Geosciences Museum of the Federal University of Pará represents Marcos Paro’s Zn-Pb ore, and is composed of ferrous sphalerite and galena, as well as pyrrhotite, amorphous silica, chlorite, phengite, Ca-phosphates, quartz and carbonaceous material. Galena and sphalerite occur in both coarse and fine crystalline aggregates, dispersed in the matrix of chlorite, phengite and siliceous carbonaceous material. The data presented are partial, since they were obtained only from a small aliquot of the sample.
Keywords: Sphalerite ferroan, galena, pyrrhotite, phengite, chlorite, Ca-phosphate, Marcos Paro.
INTRODUCTION
The beautiful sample donated to the Geosciences Museum by geologist Paulo Afonso Ribeira Barbosa (Santa Elina Mining Company) and prof. Dr. Roberto Vizeu (FAGEO-UFPA) in July 2019 represents the Zn-Pb ore of the Target DM-1, Mina Marcos Paro, in the municipality of Nova Brasilândia D´Oeste, State of Rondônia (Figure 1 A). The sample was filed under number 2478 in the collection of the Geosciences Museum of the Federal University of Pará.
ANALYTICAL METHODS, RESULTS AND DISCUSSIONS
The sample was then sectioned into two parts, and the cutting surfaces were carefully polished (Figure 1 B). In this way you have a natural view of the broken and polished surface, which allows you to also evaluate the aspects of the metallic shines of their minerals, mostly sulphides of Zn, Pb and Fe. The polished surface became beautiful, too.
X-RAY DIFFRACTION MINERALOGY
The sample was then submitted to X-ray diffraction analysis (D 2 Phaser Bruker Diffractometer) (Figure 3). The minerals identified were: ferrous sphalerite, galena and pyrrhotite, in decreasing order of abundance, sphalerite and galena, respectively the ores of Zn and Pb. The pyrrhotite confers magnetism to the sample. Phengite, chlorite and possibly crandallite (?) were still determined.

Figure 2 – XRD analysis of the Museum collection sample Nr. 2478, Zn-Pb ore from Mina Marcos Paro. Legend: Chl (chlorite); Phg (phengite); Cra (crandallite); Gln (galena); Sph (sphalerite); Pyr (pyrrhotite).
CHEMICAL COMPOSITION BY XRF portable (Bruker S1 Turbo)
The bulk chemical composition analyzes however semi-quantitative results (Table 1) demonstrate the chemical nature of the ore, dominated by Zn and Pb sulphides, as well as iron, respectively sphalerite, galena and pyrrhotite. But furthermore, they show the presence of variable and sometimes expressive concentrations of SiO2, Al2O3, CaO and K2O and even MgO (not indicated in the table due to the very high uncertainty of the method), are in agreement with the presence of phengite and chlorite, identified by DRX, too. On the other hand, P2O5 levels ranging from 1.6 to 4.37% are surprising. By XRD it was inferred mineral from the crandallite group (Ca, Pb), however, although the chemical analyzes show expressive CaO content, it was not always found a positive relationship between the values of this element and Pb with those of P2O5. It is likely that it may contain Fe and / or Al phosphates, or simply Ca phosphate. It is necessary to intensify and refine XRD mineralogical analyzes.
Table 1 – A chemical composition (by Bruker portable FRX) of Zn-Pb ore from Mina Marcos Paro, Nova Brasilândia do Oeste-RO, Santa Elina Ore Company (Geoscience Museum collection Nr. 2478).
| Analyzes | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Al203 | 19,7 | 4,8 | 3,3 | 3,9 | 8,5 | 9,5 | 5,4 | 3,8 | 8,66 | 6,0 | 5,19 | |
| SiO2 | 15,5 | 62,1 | 3,5 | 8,3 | 3,7 | 20,1 | 49,9 | 3,2 | 3,35 | 6,2 | 4,03 | |
| P205 | 2,72 | 3,9 | 1,66 | 3,3 | 2,9 | 4,1 | 2,48 | 1,79 | 4,03 | 4,05 | 4,37 | 2,48 |
| S | 27,6 | 41,8 | 21,9 | 38,5 | 38,5 | 44,8 | 35,2 | 27,7 | 47,9 | 45,8 | 33,5 | 41,6 |
| K2O | 1,4 | 2,8 | 1,16 | 0,01 | 0,47 | 0,08 | ||||||
| CaO | 4,9 | 2,9 | 0,24 | 2,1 | 0,07 | 0,25 | 0,65 | 3,84 | 0,28 | |||
| MnO | 0,16 | 0,26 | 0,147 | 0,19 | 0,42 | 0,22 | 0,22 | 0,21 | 0,3 | 0,21 | 0,23 | 0,24 |
| Fe2O3 | 15,8 | 20,8 | 14,7 | 17,5 | 48 | 44,3 | 20,3 | 15,5 | 68,8 | 42,7 | 23,5 | 23,2 |
| Cu | 1,2 | 0,23 | 0,48 | 0,16 | 0,26 | 0,03 | 0,13 | 0,84 | 0,1 | 0,03 | 0,21 | 0,59 |
| Zn | 11,0 | 45,1 | 8,07 | 27,8 | 14,9 | 2,9 | 20,5 | 13,2 | 9,7 | 3,02 | 15,0 | 31,1 |
| As | 2,83 | 0,8 | 0,43 | 0,66 | 0,69 | 1,14 | 0,51 | 0,35 | 0,1 | 2,2 | 1,2 | |
| Pb | 13,9 | 3,88 | 0,17 | 3,2 | 2,61 | 3,14 | 0,4 | 0,9 | 8,12 | 2,14 | ||
SEM MICRO-MORPHOLOGY AND TEXTURES; EDS MINERAL CHEMICAL COMPOSITON
Analyzes by scanning electron microscopy with energy dispersive x-ray fluorescence spectrometry (SEM / EDS, HITACHI / TM 3000 / Oxford Instruments / SwiftED3000; non-metallized sample and low vacuum) confirm the presence and content range of the elements obtained by Portable XRF and mainly also reinforce the presence of Mg and P2O5. And thus, attests to the presence of chlorite, probably Fe-Mg-Al, and Ca phosphate, which is apparently not apatite (not yet identified in the first x-ray diffractograms), the possibility for monetite, Ca (HPO4). This confirms the consistent levels of phosphorus in the small whole sample.
SEM / EDS analyzes also show that sphalerite does contain Fe (sphalerite ferroan), ranging from 5 to 20% of Zn content, which corresponds to 7 to 14% of Fe (Table 2), however a good portion of total iron may be represented by pyrrhotite and chlorite.
Table 2 – Chemical composition of ferroan sphalerite “CRYSTALS” of Zn-Pb ore from Marcos Paro after SEM/EDS.
| Analyzes | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Carbon | 10.216 | 7.686 | 7.141 | 9.264 | 6.570 | 6.508 | 6.458 |
| Oxygen | 1.905 | 2.677 | – | – | 3.323 | 2.903 | – |
| Aluminum | 0.350 | – | – | – | – | 0,335 | – |
| Silicon | 0.599 | 0.518 | – | 0.330 | 1.121 | 0.929 | 0,496 |
| Sulfur | 27.222 | 26.408 | 28.686 | 27.334 | 26.738 | 27.633 | 30.200 |
| Iron | 9.749 | 7.225 | 8.787 | 14.700 | 10.917 | 6.889 | 8.058 |
| Zinc | 49.959 | 54.724 | 55.385 | 48.373 | 51.331 | 54.803 | 54.789 |
Galena masses are chemically homogeneous according to these same analyzes (Figures 3 and 6) when in coarse and cleavable grains, while fine grains are dispersed in chlorite (Figure 3), amorphous silica (Figures 4 and 5), in the same way as sphalerite, and partly pyrrhotite. Pyrrhotite is often fine grains (Figures 7 and 8). The intergrowth between the amorphous silica matrix and carbonaceous matter, generally containing tiny sphalerite and partly galena granules, and even pyrrhotite. But sphalerites usually contain or are intergrown with carbonaceous organic matter (Table 2 and figures 4 and 5).
Chlorites tend to be ferrous, partly magnesian, partly alumina, and are also associated with carbonaceous organic matter and the sulfides of Zn and Pb (Figure 3). Phengite occurs locally (Figure 8).
Ca-phosphate, probable monetite, was inferred from the chemical analyzes of the masses dominated by amorphous silica and carbonaceous organic matter. For the time being, its grains (Figures 4 and 5) are not individualized as crandallite, the latter identified by XRD.
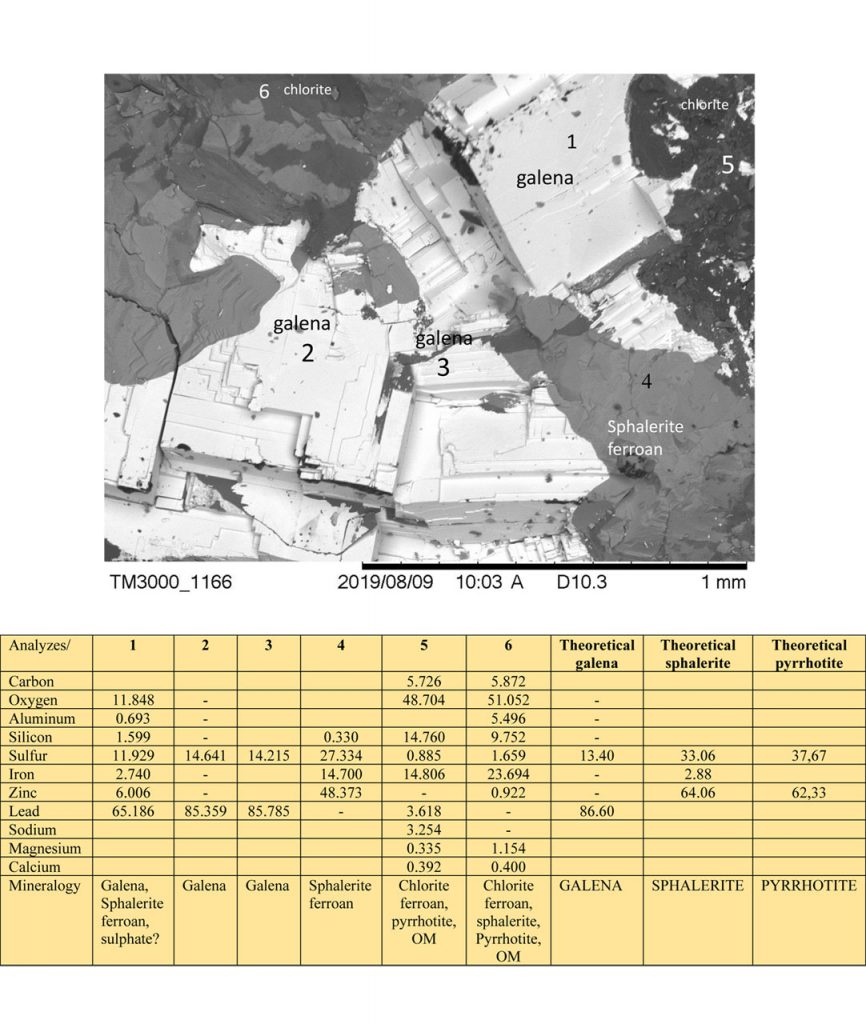
Figure 3 – SEM images and EDS chemical analyzes on mineral grains of Zn-Pb ore from Marcos Paro illustrating the composition of galena, sphalerite, pyrrhotite, chlorite and possible presence of carbon organic matter (OM) and the theoretical chemical composition of galena, sphalerite and pyrrhotite for comparison.

Figure 4 – SEM images and EDS chemical analyzes on mineral grains of Zn-Pb ore from Marcos Paro illustrating the composition of galena, sphalerite ferroan, silica amorphous, Ca-phosphate and possible presence of carbon organic matter (OM).
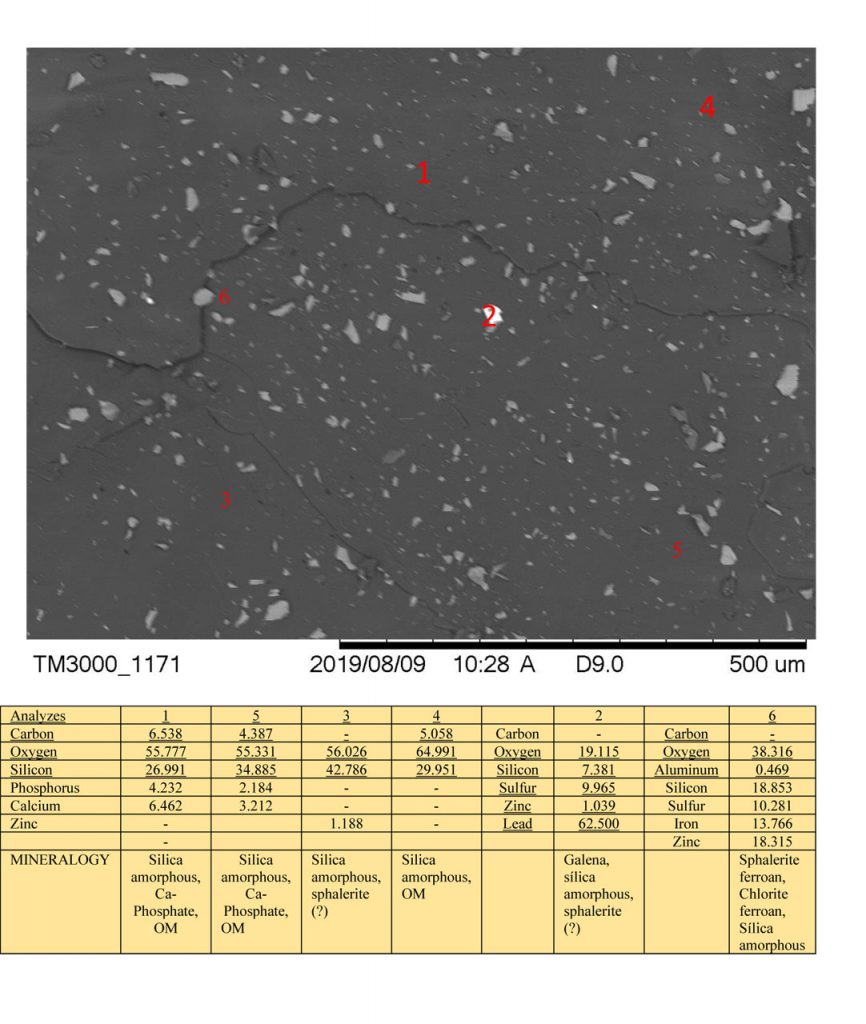
Figure 5 – SEM images and EDS chemical analyzes on mineral grains of Zn-Pb ore from Marcos Paro illustrating the composition of silica amorphous, galena, sphalerite ferroan, Ca-phosphate and possible presence of carbon organic matter (OM).
The presence of sulfates (Al and/or Fe) was inferred from the chemical analysis of EDS (Figures 3 and 6). In the sulphide domain mass, analyzed by both XRD and SEM / EDS, no quartz was detected, although SiO2 content is disconnected from Al2O3 and alkalis. These silica contents are assumed to be amorphous silica, always bound to expressive carbon contents, considered to be representative of organic material. However, quartz is visible to the naked eye in the hand sample, in pockets and venules.
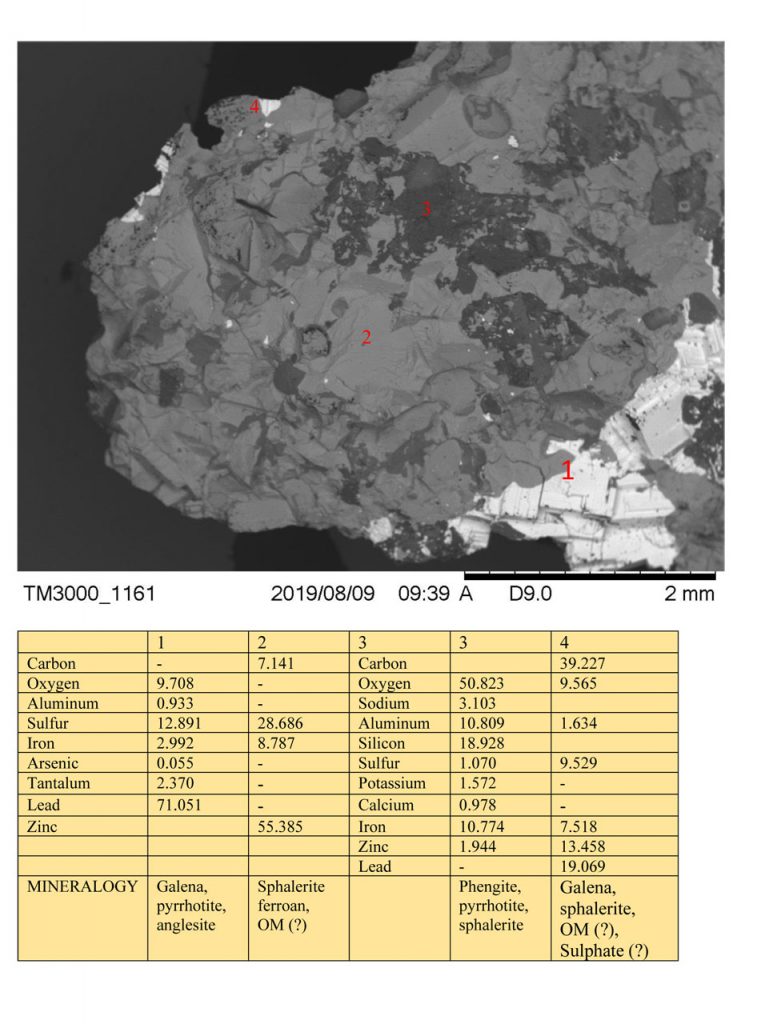
Figure 6 – SEM images and EDS chemical analyzes on mineral grains of Zn-Pb ore from Marcos Paro illustrating the composition of galena, sphalerite ferroan, pyrrhotite, sulphate minerals (?) and possible presence of carbon organic matter (OM).
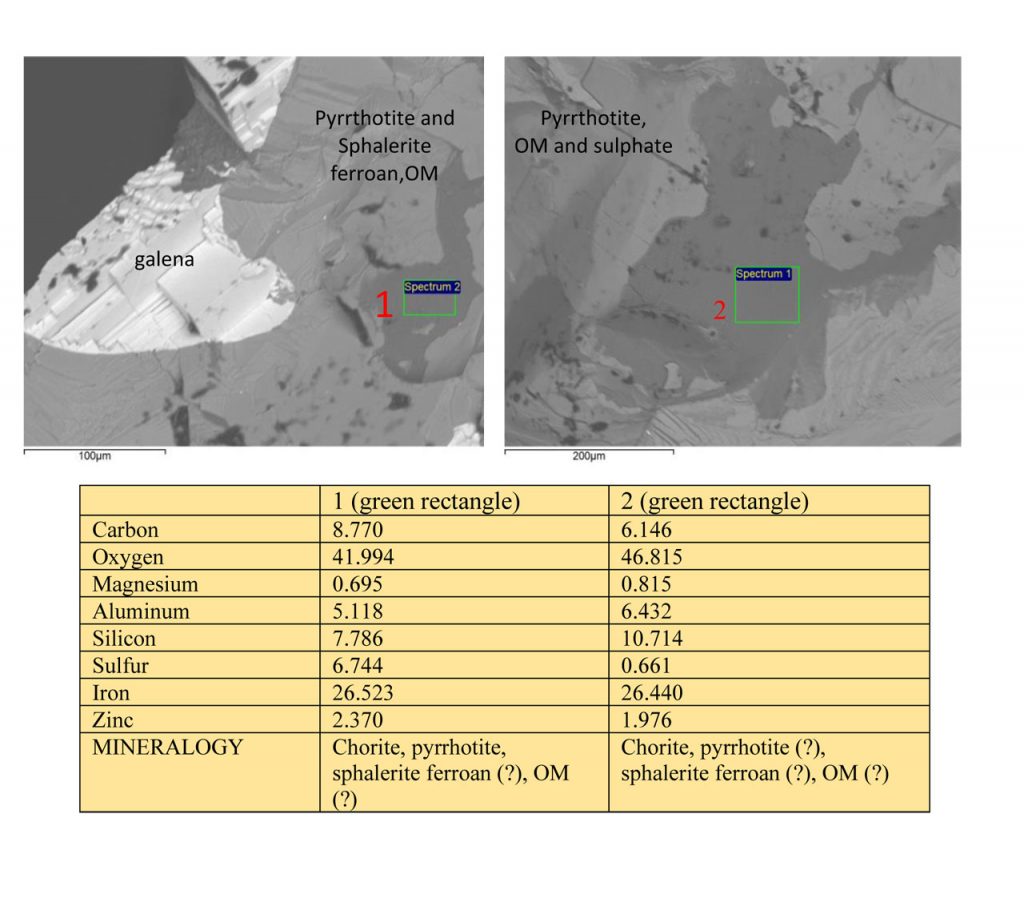
Figure 7 – SEM images and EDS chemical analyzes on mineral grains of Zn-Pb ore from Marcos Paro illustrating the composition of chlorite, sphalerite ferroan, pyrrhotite, and possible presence of carbon organic matter (OM).
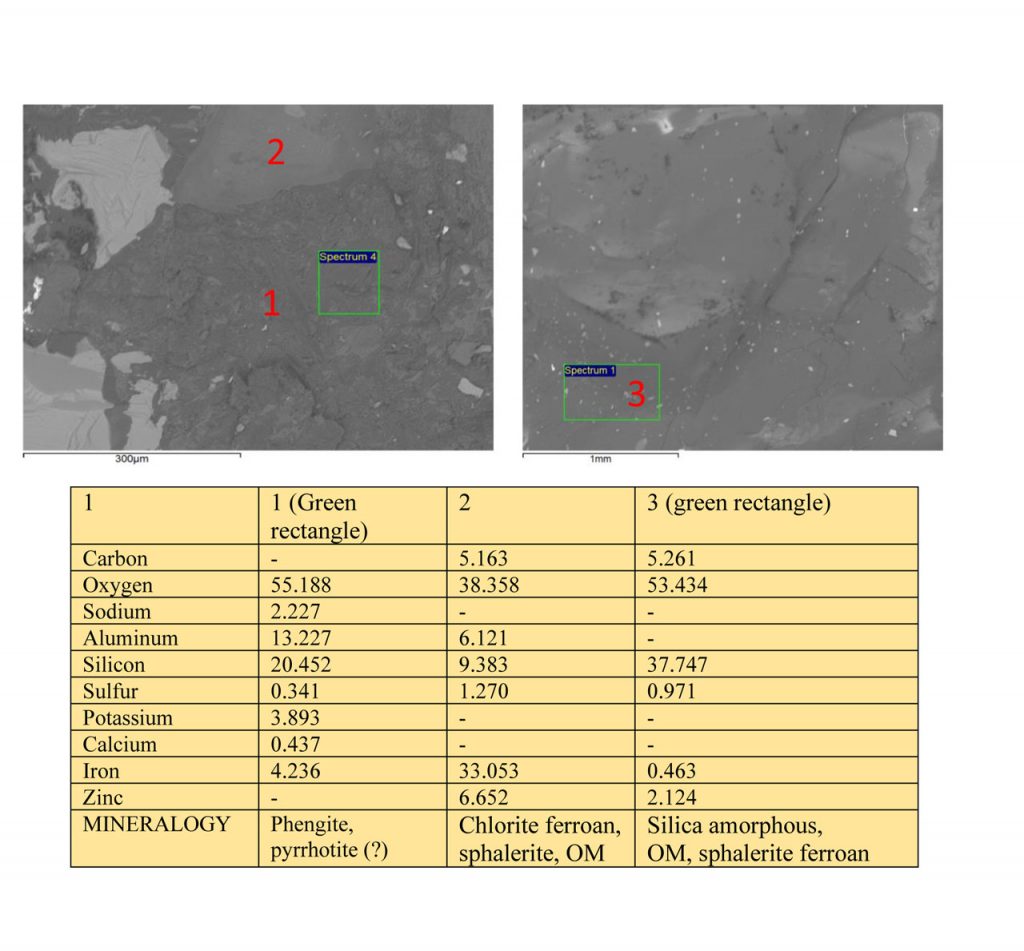
Figure 8 – SEM images and EDS chemical analyzes on mineral grains of Zn-Pb ore from Marcos Paro illustrating the composition of phengite, chlorite ferroan, silica amorphous, sphalerite ferroan and possible presence of carbon organic matter (OM).
CONCLUSIONS
Sample 2478 is an excellent and beautiful example of Zn-Pb ore to ferrous sphalerite and galena, associated with pyrrhotite, a very thin amorphous silica mass with a high content of carbonaceous material (?). Galena and sphalerite can form thick masses, but they are also very common as small grains distributed without distinction in the mass of amorphous silica, chlorite and even phengite, which may contain pyrrhotite. Quartz has been identified mesoscopically in sub centimeter pockets and/or venules aggregates as remobilizations. This complex mineralogical assembly suggests that Zn-Pb mineralization was initially associated with a sedimentary package rich in carbonaceous and siliceous organic matter, in addition to phosphates, subject to remobilization and concentration by hydrothermal action under very reducing conditions. The data obtained from very rapid and still partly semi-quantitative analysis recommend that advanced and systematic studies be implemented if they are not already in progress or even completed. Unfortunately, in a first scan without much tenacity in the literature no data related to this ore were found.
Acknowledgements
The authors thank geologists Geol. Paulo Afonso Ribeiro Barbosa of Mining Santa Elina and Prof. Dr. Roberto Vizeu for the memory of the UFPA Geosciences Museum, when they donated such a beautiful and significant mineral sample. To CNPQ for the support with productivity scholarship and grant (Proc. 305015/2016-8) and to PNPD-PPGG for granting PDR scholarship to the second author. All analyzes were performed at the LAMIGA IG / UFPA laboratories.

