02 – A FLUOR-POOR WAVELLITE IN PHOSPHATE-RICH IRON CRUSTS IN AMAZON
Ano 07 (2020) – Número 01 Artigos
![]() 10.31419/ISSN.2594-942X.v72020i1a2MLC
10.31419/ISSN.2594-942X.v72020i1a2MLC
Marcondes Lima da Costa1*, Alessandro Sabá Leite2, Ronny Kaden3, Herbert Poellmann3, Nilson F. Botelho4, Daniel Chaves Santos5
1Instituto de Geociências (IG), Universidade Federal do Pará, Belém, Brazil, marcondeslc@gmail.com, mlc@ufpa.br
2PPGG/IG, Universidade Federal do Pará, Belém, Brazil, alessandro.leite@me.com
3Universität Halle-Wittenberg, Halle an der Saale, Germany, herbert.poellmann@geo.uni-halle.de
4 Instituto de Geociências, Universidade de Brasília, Brasília, Brazil, nilsonfb@unb.br
5Phosfaz, Bonito, Pará, daniel.chaves@phosfaz.com.br
*Corresponding author
ABSTRACT
The minerals of the alunite supergroup, which comprises aluminum phosphates and sulphates (APS), which in turn constitute the groups/series of crandallite-goyazite, woodhouseite-svanbergite and wardite-millisite, involve more than forty minerals, which are formed under the hydrothermal to supergenic conditions. Among the supergenics are the lateritic formations, being the Gurupi region, or more precisely Northeast of Pará and Northwest of Maranhão, in the Eastern Amazon a world classic geological example. Here, in addition to the minerals of these groups or series, senegalite, augelite, variscite and also wavellite occur. The latter, however, is always restricted, while the others may form a characteristic horizon. At the Sapucaia phosphate mine in Bonito, Pará, this mineral is presented in beautiful centimeter druses. The work describes this mineral in terms of its morphological, crystallographic and chemical characteristics, highlighting its poverty in fluorine, and discusses why its formation is restricted in terms of volume and to aluminous phosphate iron crust, concluding that the wavellite is directly linked to the initial weathering alteration.
Keywords: lateritic iron crust; crandallite-goyazite; woodhouseite-svanbergite; Sapucaia; Bonito; Gurupi.
INTRODUCTION
The geological occurrences of aluminum phosphates are frequent in the Earth’s crust, normally associated with peraluminous rocks in different environments (Dill, 2001), and are currently denominated together with aluminum sulfates, APS (Aluminum Phosphates and Sulfates Minerals), with which are generally associated. APS comprises more than 40 species in the alunite supergroup alone (Dill et al., 1995, Jambor, 1999; Dill, 2001. The general formula of these alunite minerals is AB3(XO4)2 (OH)6, where A is a large cation (Na, U, K, Ag, NH4, Pb, Ca, Ba, Sr, REE) and B sites are occupied by cations of the elements Al, Fe, Cu and Zn. In nature, the anion (XO4)x- is dominated by P and S (Dill, 2001). The alunite supergroup comprises the series of crandallite, woodhouseite and wardite, among others. In the series of crandallite the most important minerals are, this mineral, and goyazite, florencite, gorceixite and plumbogummite; in the woodhouseite, this mineral and svanbergite; in the wardite series, wardite and millisite. The minerals from these series are frequent in lateritic aluminum phosphate deposits, with the Northeast region of Pará and Northwestern Maranhão (Costa & Costa, 1980; Costa et al., 1980, Oliveira & Schwab, 1980; Schwab et al., 1983, 1989, 1993; Pöllmann et al., 1987; Toledo et al., 2006; Costa et al., 2016), as the most significant example worldwide. Dill (1995, 2001) shows the wide range of environments in which these minerals can form and, therefore, demonstrates how frequent they are. In general, they are found as small occurrences, many of them even rare, associated with hydrothermal veins, in rocks such as phyllites and schists, mainly as venules and quartz veins; also in rocks modified by volcanic activities (Dill et al., 1995) and in tropical and paleosol soils, in cryptocrystalline, spherulite and acicular-radial and microcrystalline masses, and rarely as millimeter crystals in cavities. In addition, aluminum and aluminum-iron phosphates are known in recent organic accumulations, such as the guano deposits in Chile and Peru (Costa, 1979, 1982) and old ones in Romania in caves developed in limestone terrains (Costa, 1982; Polyak et al., 1996; Dill, 2001). In lateritic environments, complex solid solution series of these minerals occur, as already mentioned, but in addition to them, variscite, AlPO4.2H2O, wavellite, Al3 (PO4)2 (OH, F)3•5(H2O), augelite, Al2(PO4) (OH)3 and more recently Senegalite, Al2(PO4) (OH)3•(H2O) (Johan, 1976; Costa & Reymão, 1984; Pöllmann et al., 1987) also stand out. These aluminum phosphates, however, can form economical mineral deposits in lateritic formations (Costa et al., 2016). The main lateritic occurrences in Senegal, the Christmas Islands, Australia, Ghana, Florida and Brazil (Capedcomme, 1953; Slansky et al., 1964; Flicoteaux, 1982; Costa,1982; Flicoteaux & Lucas, 1984; Dill, 2001) formed mainly from sedimentary rocks with phosphorites; in Canada, Australia, Gabon and Brazil (Costa, 1979, 1982; Schwab et al 1993) from magmatic rocks mineralized in apatite, and metamorphic in Brazil, Ghana, (Costa, 1979, 1982). In Brazil alone, lateritic aluminum phosphates have found a favorable environment to form, namely: exposure of in apatite mineralized primary rocks to hot and humid tropical conditions during the Cenozoic, which are essential for the establishment of intense tropical weathering, and in the Amazon region they reached their climax. Laterite formations mineralized in aluminum phosphates have been discovered in several areas of this region, however the biggest highlight is for the eastern portion of this region, the Northeast of Pará and the Northwest of Maranhão (better known as the Gurupi region, Figure 1), in its Atlantic coast zone and not so much from it (Costa, 1979, 1982; Costa & Costa, 1980; Costa et al., 1980; Oliveira & Schwab, 1980; Schwab et al., 1983, 1987; Costa et al., 1980, 2016), as previous mentioned. The first publication with reference to bauxite with phosphorus in the region (Pirocaua and Trauira) was presented by Shaw et al. (1925) and the first detailed pioneering publication on aluminum phosphates in the region by Brandt (1932), who recognized the lateritic terrain, but did not recognize the phosphates as lateritic, but as guano deposits.
In this region, aluminum phosphates constitute the upper portion of the lateritic profile, forming a horizon up to more 10 m thick, extending up to 1.5 km in length. The most common aluminum phosphates are from the crandallite-goyazite, augelite, senegalite, wardite and variscite series. Wavellite is present, but always very restricted. Augelite and senegalite were found as mm crystals lining sub-centimeter cavities, but in general they are massive and constitute in some deposits a thick horizon rich in phosphorus. By the way, senegalite was an unknown mineral until 1976, when it was discovered in Senegal (Johan, 1976) as a rarity and later as an abundant mineral in the Gurupi region (Costa & Costa, 1980; Costa et al., 1980; Costa & Reymão, 1987; Pöllmann et al., 1993). Wavellite, on the other hand, seems much more restricted, does not develop a mineral horizon, like the others. It has recently been identified in millimeter to sub-millimeter-large crystals in cavities up to 20 cm inside of the aluminum phosphate iron crust from the Sapucaia phosphate deposit in Bonito, in the northeast of Pará (Costa et al, 2016, in this BOGEAM bulletin). On this occasion, the present work describes and discusses this occurrence in the context of lateritic deposits in the Gurupi region, but especially in the Phosphorues Rectangle “Santa Maria-Bonito-Ourém-São Miguel do Guamá”, in the northeast of the state of Pará (Figure 1). In fact, the current knowledge is a result of the pioneering research work of the Phosphates Project in the Northeast of Pará and the Northwest of Maranhão, financed by the Humid Tropic/CNPQ, started in 1976 with the active participation of the first author, since that time, and coordinated by the UFPA professor Manoel Gabriel Siqueira Guerreiro, already deceased, who unfortunately was not thrilled with the theme.
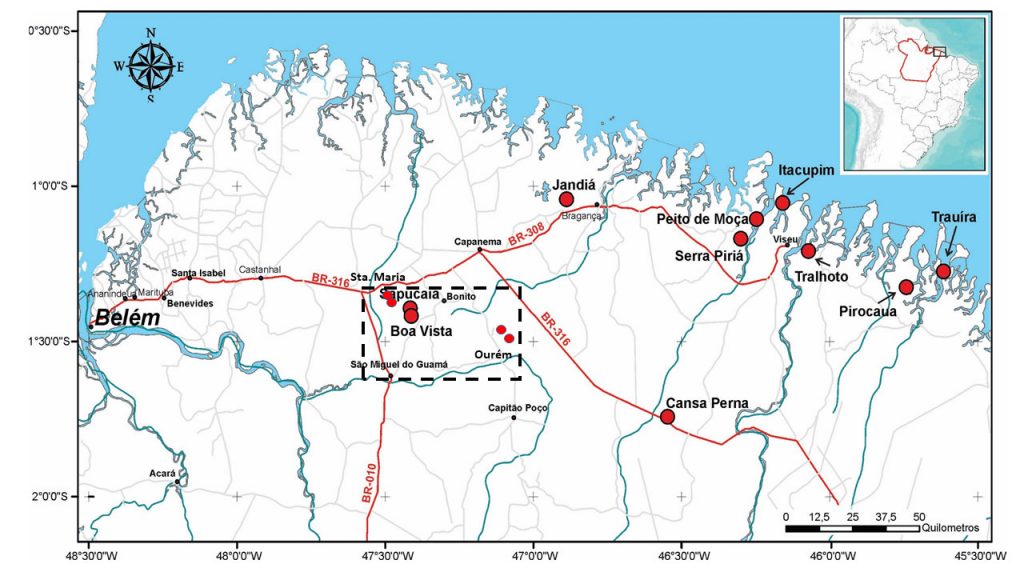
Figure 1 – Location of the Sapucaia aluminum phosphate deposit, in the municipality of Bonito, northeast of the state of Pará and of the other known deposits in northeast of Pará and northwest of Maranhão (Gurupi region), with emphasis on the Rectangle Santa Maria-Bonito-Ourém – São Miguel, highlighting the most recent discoveries of aluminum phosphates in this area (smaller dots in red). Modified from Leite (2014).
The phosphates from Sapucaia, at Bonito
The phosphates of Sapucaia and Boa Vista deposits are located in the municipality of Bonito, in the northeast of the state of Pará, 140 km far from its capital Belém (Figure 1). These deposits were discovered and studied by Costa & Costa (1987, 1991) and investigated in detail by Leite (2014), Costa et al. (2016) and Santos (2019). Research aimed at the economic use of phosphorus as fertilizer was carried out by the companies Fosfatar, Rio Verde Minerals, Vicenza Mineração and Phosfaz. They conducted geological, geophysical and shallow drilling surveys, which made it possible to delimit the ore body and started the installation of equipment and industrial facilities for extraction, processing and production of thermophosphates in loco. The mining was repeatedly postponed, but finally became a reality in 2015, aiming to meet the region’s demand. At the same time, Phosfaz developed research in search of new deposits, and found four more (Serrote, Serrotinho, Tracuá and Caeté) (Santos, 2019) (Figure 1), but all small, although in geology similar to Sapucaia and Boa Vista. The main deposit, Sapucaia, mirrored in a smooth and small plateau, has an elongated north-south shape and reaches 650 m in length (Figure 2). The maximum altitude is 80 m. This plateau surface is supported by an iron-aluminum-phosphate crust, jaguar skin-like type and partly by phosphate crust.
The aluminum phosphate bodies of Sapucaia and Boa Vista, representing a classic full lateritic and erosively truncated lateritic profile, mainly in Boa Vista (Leite, 2014; Costa et al., 2016), are in the domain of sedimentary geological terrains of Paleozoic age represented by the Guamá sandstones, siltite and sandstones of the Itapecuru Formation, Cretaceous in age (Parnaíba Basin) and the calcitic and marl limestones of the Pirabas Formation of Lower Miocene age, generally obliterated by the Miocene and Pleistocene siliciclastic sediments of Barreiras Formation/Group, which extends over much of the region (Figure 2). Drilling holes reached these limestones around the Boa Vista deposit. These sedimentary rocks find as substrates diverse proterozoic granitic bodies and paleoproterozoic granite-gneissic terrains and phyllite and schist sequences (Piriá, Piritoró, Jeritequara and Vila Cristal Formations), generically not long ago denominated Gurupi Group (Figure 2).
The lateritic body of the Sapucaia phosphate deposit (Figure 3) displays a lower, clayey, sometimes kaolinic horizon, which emerges strictly on the plateau slopes, but which has been reached by the drilling holes. It is made up of kaolinite, quartz, muscovite, aluminum phosphates and goethite / hematite. This horizon is in gradual and undulating contact with the aluminum phosphate horizon upwards. The phosphate horizon is compact, microcrystalline, hard, porous, white to yellow in color and its thickness varies between 0.20 and 9 m. It tends to form a crust. It consists of crandallite-goyazite, wardite, woodhouseite-svanbergite, quartz and kaolinite, partly muscovite / illite (Figure 4). The top of the aluminum phosphate horizon goes locally into an aluminum-phosphate iron crust, formed by reddish centimeter-large nodules of hematite cemented by aluminum phosphates, the same as those of the phosphate horizon (Figure 5), which at the top assumes the classic jaguar-skin pattern. Small cavities in this crust are locally coated and/or filled with millimeter to sub-millimeter-large wavellite crystals (Figure 5 D), the main goal for this study.
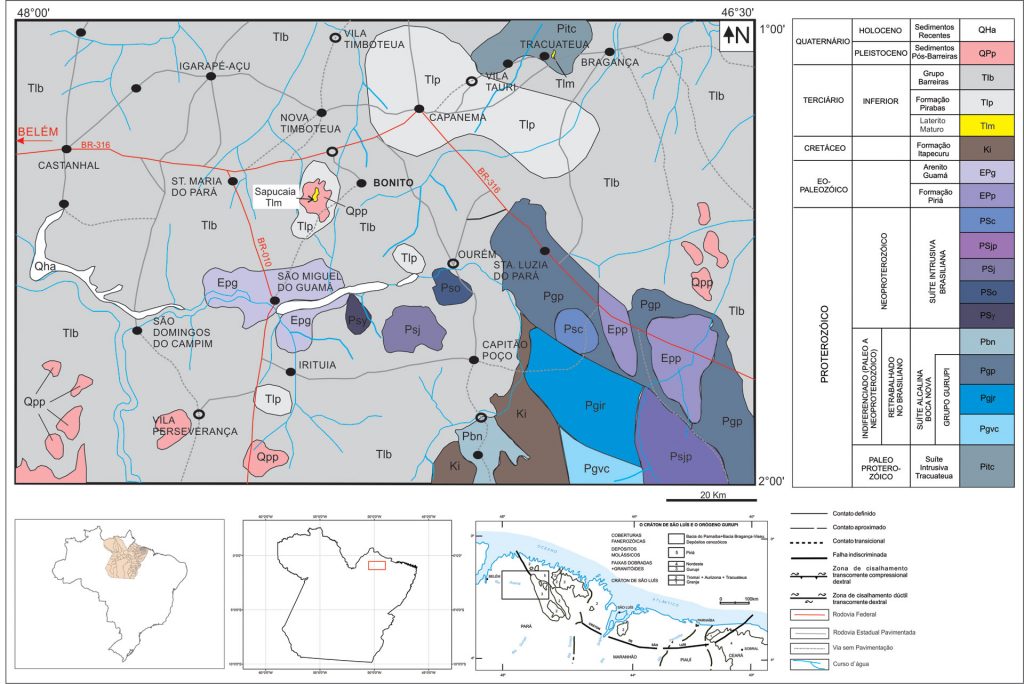
Figure 2 – Geological map from region being at the central part the phosphor rectangle Sta.Maria-Bonito-Ourém-São Miguel showing near to the center the Sapucaia phosphate deposit at Bonito. Modified after Costa (2000) and Leite (2014).

Figure 3 – Geological lateritic horizons identified in the phosphate deposit of Sapucaia, at Bonito. Modified after Leite (2014).
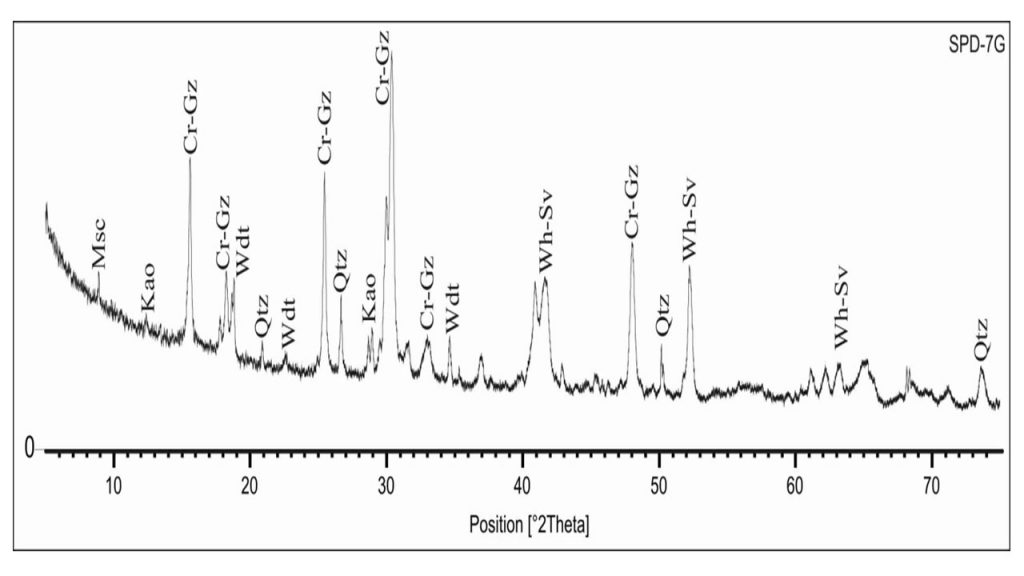
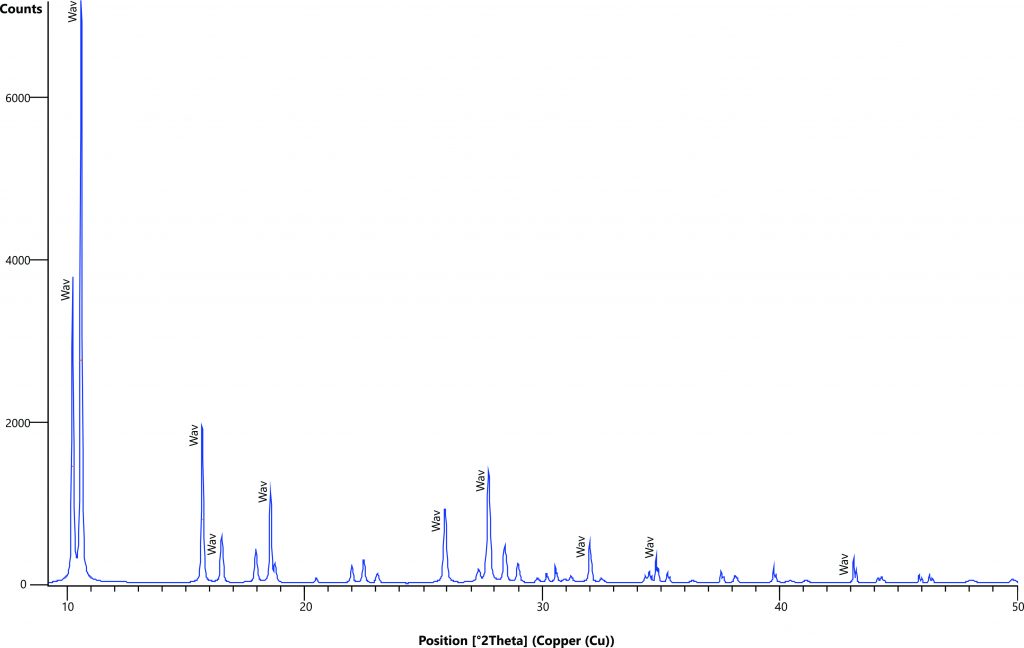
Figure 4 – XRD analysis of a typical aluminum phosphate iron crust showing crandallite-goyazite (Cr-Gz), woodhouseite-svanbergite (Wh-Sv), wardite (Wdt), kaolinite (Kao) and quartz (Qtz). After Leite (2014).

Figure 5 – Some geological aspects of the aluminum phosphate deposit of Sapucaia: A) The open pit mine for extraction of phosphates; B) the typical aluminum phosphate ore constituted mainly by crandallite-goyazite; C) the typical aluminum phosphate iron crust showing the jaguar skin aspect, which locally presents the small druses of wavellite; D) detail of aluminum phosphate iron crust showing some wavellite druses (red arrows).
The aluminum phosphate horizon and the aluminum phosphate iron crust constitute the high-grade ore. Table 1 gives an idea of the chemical composition of this ore, the poor and the iron rich one. As already seen by the mineralogical constitution, they are made of calcium and strontium phosphates and in part aluminum sulphates, with 18 to 27% P2O5.
Table 1 – Chemical composition (Wt. %) of some samples of the aluminum phosphate ore in Sapucaia, showing both iron-rich and iron-poor. The data were obtained by ICP-OES, XRF and loss of ignition. Modified from Leite (2014).
| Sample | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | MnO | SrO | SO3 | LOI | |
| SPD-7A | 9,00 | 26,35 | 8,68 | 0,10 | 6,86 | 0,07 | 0,07 | 1,21 | 23,47 | 0,00 | 5,58 | 1,52 | 18,3 | |
| SPD-7D | 5,55 | 24,73 | 19,13 | 0,05 | 5,66 | 0,03 | 0,05 | 1,86 | 19,41 | 0,01 | 4,42 | 2,57 | 18,7 | |
| SPD-7B | 12,42 | 22,93 | 19,29 | 0,06 | 5,31 | 0,05 | 0,11 | 1,55 | 18,10 | 0,00 | 3,71 | 1,62 | 16,1 | |
| SPD-7C | 21,09 | 27,82 | 3,61 | 0,12 | 5,72 | 0,10 | 0,39 | 0,72 | 20,15 | 0,01 | 5,11 | 1,02 | 15,0 | |
| SPD-7E | 2,16 | 33,54 | 1,75 | 0,03 | 9,86 | 0,18 | 0,06 | 0,90 | 27,09 | 0,00 | 4,17 | 4,62 | 20,1 | |
| SPD-7F | 2,78 | 32,00 | 2,22 | 0,06 | 9,62 | 0,05 | 0,00 | 1,34 | 27,74 | 0,01 | 4,82 | 2,74 | 19,1 | |
| SPD-7G | 12,79 | 27,44 | 4,30 | 0,11 | 6,78 | 0,07 | 0,10 | 0,40 | 24,29 | 0,00 | 5,91 | 2,07 | 17,0 | |
MATERIALS AND METHODS
The investigated samples were subtracted from the aluminum-phosphate iron crust with druses (up to 20 cm ø) formed by millimeter wavellite crystals. Still in the cavities, the crystals were photographed with the aid of a stereomicroscope coupled with a digital camera. Then, they were removed one by one for mineralogical and chemical analyzes. Mineralogical analyzes were performed by DRX (IG/UFPA laboratories with Bruker equipment, in Belém, Pará; and Universität Halle-Wittenberg, Panalytical equipment; by DTA/TG DTG at the same Universität, Germany. Semi-quantitative images and chemical analyzes were carried out by SEM/EDS at IG/UFPA, while quantitative chemical results from the same crystals were obtained with the aid of an electronic microprobe from the Institute of Geosciences of the University of Brasília.
RESULTS
Occurrence and morphology of wavellite crystals
Wavellite is found casually in the aluminum phosphate iron crusts of Sapucaia, when these are apparently partially chemical dismantled (Figure 6). With the intensification of mining, new beautiful specimens were exposed. Wavellite druse developed in small cavities (up to 20 cm large) inside of an aluminum phosphate iron crust presenting a jaguar skin pattern. The reddish nodules are made of hematite (if it becomes a little brown it may also contain Al-goethite and Fe-goethite black films) and some dispersed calcium-strontium aluminium-phosphate, while the yellowish to whitish cement crandallite-goyazite, mainly. The druses preferentially have been developed in the phosphate cement.
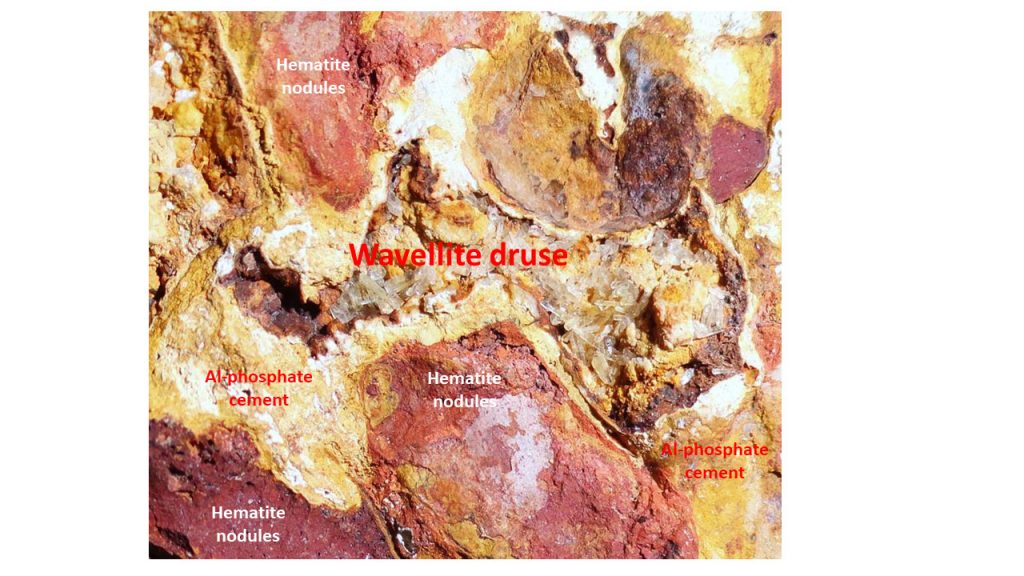
Figure 6 –Wavellite druse developed in small cavities inside of a typical lateritic aluminum phosphate-rich iron crust presenting a jaguar skin pattern. The reddish nodules are made of hematite (when a little brown also by Al-goethite, besides black films of Fe-goethite) and some aluminum phosphates, while the yellowish cement crandallite-goyazite, mainly.
Wavellite crystals are generally sub-millimetric, euhedral, elongated along the c axis, with the development of the prisms {110}, {010} and pyramid {011} and / or {111}; they are colorless and transparent and striated // c (Figure 7).
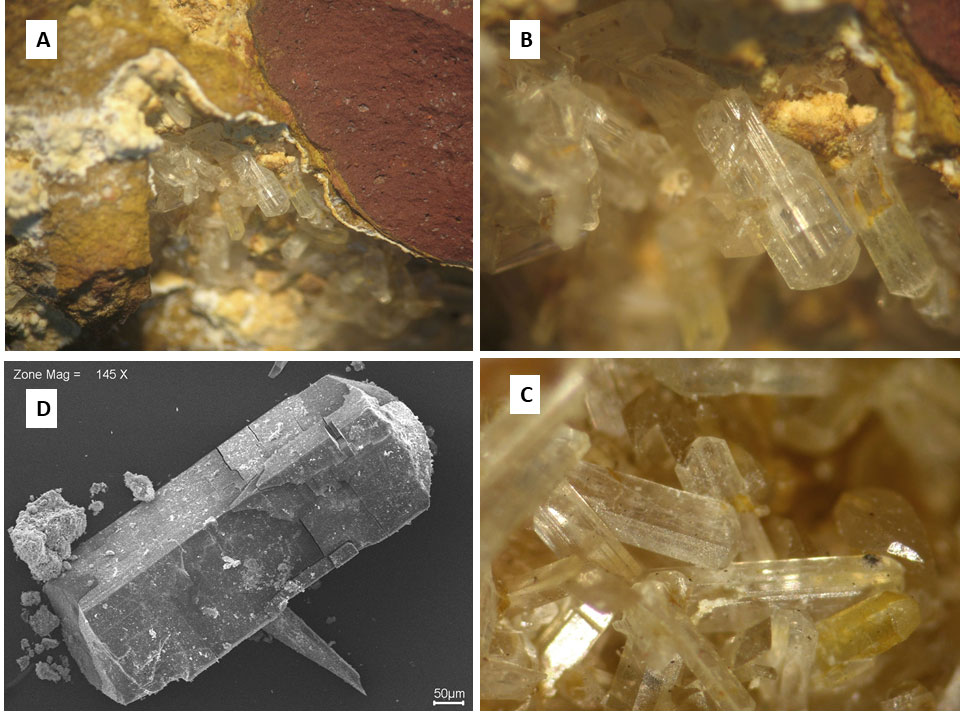
Figure 7 – Wavellite crystals in druses developed inside of the aluminum phosphate iron crust from Sapucaia. A) a segment of a druse inside of aluminum phosphate matrix close to hematite nodule; B) detail of some striated crystals; C) another aspect of the crystals: D) SEM image of a wavellite crystal.
XRD spectrum, crystallographic parameters and thermal behavior
The XRD results (Figures 8 and 9 A) clearly show the wavellite composition. Its crystallographic parameters are shown in Table 2 and were compared with Freihung’s fluorine-rich wavellite, according to data by Kaden et al. (no date). The small differences between qualifying crystallographic and physical data are evident, certainly a consequence of the divergences in fluoride contents. The DTA analyzes show that the free individual crystals from Bonito (Figure 9 B) start slightly lose weight when heated accomplishing this first dehydration at 62 °C with an endothermic DTA signal at 60 °C (Figure 9 B). Although similar in stoichiometry and structure, for crystals near the wall of the druse this dehydration step is finished at 70 °C with an endothermic DTA signal at 66 °C (Kaden et al., no date).
With decreasing fluoride contents TDTA and H2Ocum are decreased from 157 °C, 5.0 H2O (Freihung) towards for the Bonito wavellites 136 °C, 3.9 H2O or 144 °C, 3.8 H2O (Kaden et al., no date).
Table 2 – Crystallographic parameters for wavellite from Sapucaia, at Bonito, compared to that F-rich one from Freihung. After Kaden et al. (no date)
| Characteristics | Bonito, needle | Bonito, slice | Freihung |
| Fluoride (apfu), EMPA | 0.33 | 0.22 | 0.98 |
| Molar weight (g/mole) | 412.64 | 412.42 | 413.93 |
| a (Å) | 7.0209(5) | 7.0285(5) | 7.0005(5) |
| b (Å) | 17.3774(12) | 17.3970(12) | 17.3671(11) |
| c (Å) | 9.5916(7) | 9.5934(7) | 9.6342(6) |
| V (ų) | 1170.23(24) | 1173.03(24) | 1171.30(22) |
| ρcalc (g/cm³) | 2.3421 | 2.3353 | 2.3473 |
| µ (mm-1) | 0.698 | 0.695 | 0.701 |
| F(000) | 840.99 | 841.82 | 841.62 |
| crystal shape | needle | slice || (100) | needle |
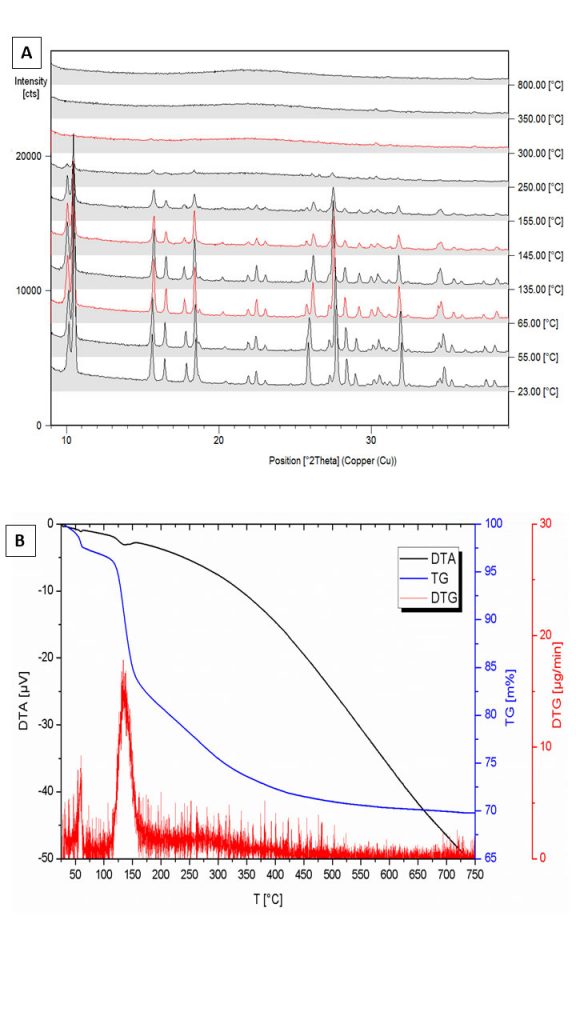
Figure 9 – (A) XRD analyzes of wavellite crystals after increasing temperature (from 25 through 800 ° C): (B) DTA, TG and DTB analyzes of wavellite crystals from Sapucaia, at Bonito. After Kaden et al. (No date).
Chemical composition
The results obtained by SEM/DS show a chemical composition compatible in part with that of theoretical wavellite, however with the presence of 2.9 to 3.9% carbon (Figure 10). In general, wavellites carry fluoride.
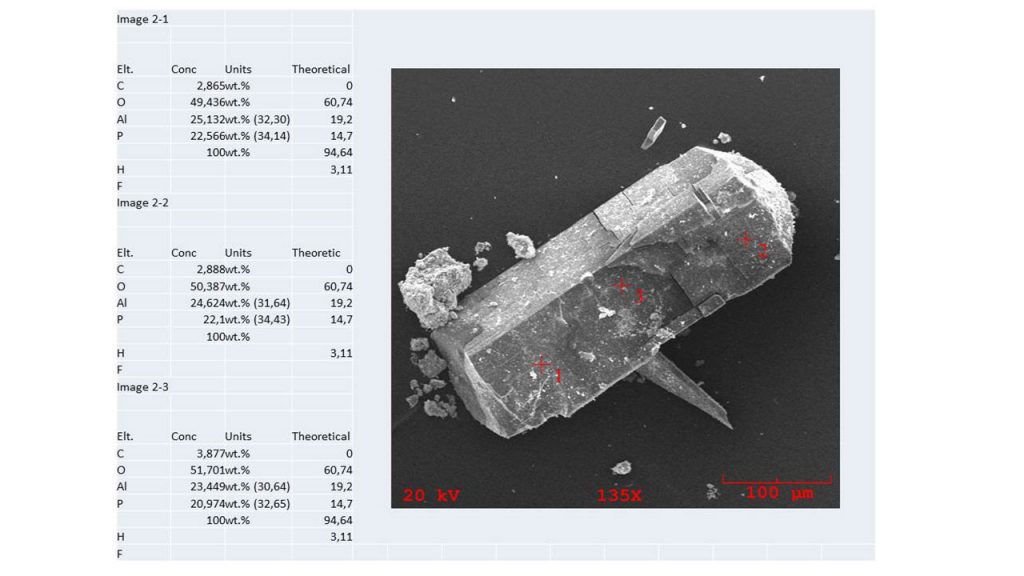
Figure 10 – SEM/EDS chemical analyzes in three points over wavellite crystal face (110). In parenthesis are the recalculated data for oxides at 100 Wt.%.
The chemical composition obtained by Electron Microprobe (Table 3) also partially diverges from that of theoretical wavellite, with higher values for both P2O5 and Al2O3. The difference found in part was debited to the fact that the analyzes were carried out on the prism faces {110}, without previous polishing, and that due to their crystallographic nature they were not positioned horizontally, consequently. On the other hand, the values obtained by SEM/EDS and recalculated for oxides and considering H2O = 27.78 (Table 3), are closer to those of the theoretical wavellite (Figure 10), but only for P2O5. The theoretical wavellite, however, still contains 2.25 Wt. % fluorine, which equals -0.95% -O = F2 Webmineral.com (accessed on April 6, 2020). In general, wavellites can vary in fluoride content according to their occurrence (Kaden et al., no date). Fluoride was not detected by a microprobe or by SEM/EDS in Sapucaia wavellite. Chemical constitution problems and consequently structural changes were identified by Kaden et al. (no date; not published yet) when studying and simulating the crystalline structure of Bonito’s wavellite, verifying that it is in principle a low-fluorine wavellite, which would be in accordance with the analyzes presented previously. These characteristics may explain in part the non-convergence of the chemical analyzes obtained in this work in comparison with that of the theoretical wavellite.
Table 3 – Microprobe chemical composition of wavellite crystals from Sapucaia deposit at Bonito. * By difference to 100 %; **, Wavellite, Al3(PO4)2(OH, F)3•5(H2O), with 2.25 %Wt. F, after Webmineral.com (accessed on April6, 2020).
| Wavellite Crystal . Point analyzed | P2O5
Wt. % |
Al2O3
Wt. % |
Fe2O3
Wt. % |
Total
Wt. % |
H2O
Wt. % |
| 1.1 | 37.022 | 37.327 | 0.556 | 74.905 | |
| 1.2 | 37.495 | 37.913 | 0.201 | 75.609 | |
| 1.3 | 36.885 | 38.816 | 0.242 | 75.943 | |
| 1.4 | 36.461 | 37.211 | 0.660 | 74.332 | |
| 1.5 | 36.475 | 37.574 | 0.232 | 74.281 | |
| 2.1 | 37.735 | 39.343 | 0.638 | 77.716 | |
| 2.2 | 36.676 | 39.346 | 0.320 | 76.342 | |
| 2.3 | 38.476 | 37.657 | 0.174 | 76.307 | |
| 2.4 | 35.586 | 37.415 | 0.422 | 73.423 | |
| 3.1 | 36.765 | 38.887 | 0.095 | 75.747 | |
| 3.2 | 38.35 | 39.012 | 0.057 | 77.419 | |
| 3.3 | 39.652 | 39.391 | 0.158 | 79.201 | |
| 3.4 | 37.254 | 40.715 | 0.099 | 78.068 | |
| 3.5 | 36.832 | 39.569 | 0.122 | 76.523 | |
| 3.6 | 37.875 | 39.396 | 0.174 | 77.445 | |
| 4.1 | 36.904 | 38.077 | 0.322 | 75.303 | |
| 4.2 | 37.507 | 40.158 | 0.414 | 78.079 | |
| 4.3 | 37.468 | 38.511 | 0.098 | 76.077 | |
| Minimum | 35.586 | 37.211 | 0.057 | 72.854 | |
| Maximum | 39.652 | 40.715 | 0.660 | 81.027 | |
| Sigma | 1.244 | 1.141 | 0.167 | 2.552 | |
| Average (N = 18) | 37.301 | 38.684 | 0.273 | 76.258 | 23.742 * |
| Theoretical wavellite**,
Al3(PO4)2(OH, F)3•5(H2O), |
33.68 | 36.29 | 0 | 69.97 | 27.78 |
| SEM/EDS analyzes | 33.74 | 31.53 | |||
| Analysis conditions: 15 kV e 10nA | |||||
| Standards: P: apatite; Al: Al2O3; Fe: andradite | |||||
DISCUSSIONS
Several laterite-related aluminum phosphates deposits have been recognized, already being a classic example, the Gurupi region in the Eastern Amazon, as demonstrated by the various published works (see Costa et al., 2016). The main aluminum phosphate minerals are those from the alunite supergroup (Crandallite-goyazite, woodhouseite-svangerbite and wardite-millisite) with X cations (large ionic radius: Ca, Na, Sr, Ba, Pb, REE), in addition to simply aluminum phosphates, namely augelite, senegalite and variscite, except wavellite. It, at least in the Gurupi region, when it occurs, is always subordinated to the centimeter-lage cavities, preferably within the iron crust. It does not develop a typical horizon, in which it dominates, as the others do. In the Gurupi region it was found in Pirocaua, Itacupim, Peito de Moça, Cansa Perna and Bonito (Figure 1). Its best expressions are in Pirocaua and Bonito, always filling cavities in millimeter crystals, colorless and transparent. In these cavities, especially in Pirocaua, it is associated with senegalite (Costa et al., 1980; Costa, 1982; Costa & Reymão, 1984; Pöllmann et al., 1987). Senegalite itself in its typical locality, in the aluminum phosphates deposits of Senegal, occurs only as small druses in sub-millimetric crystals (Johan, 1976). On the other hand, in Gurupi it forms a thick, almost massive horizon of Cansa Perna (Costa, 1982; Costa & Reymão, 1983; Pöllmann et al., 1987).
The field data show that wavellite, and in part augelite and senegalite, develop with a certain preference in cavities, especially within the iron phosphate crusts. This suggests that the crusts probably were partially altered with localized dissolution creating the cavities, which were later coated and/or filled with crystals, especially of wavellite, which can also be senegalite and augelite. These crusts are ancient, possibly from the Paleocene (Costa, 1982; Costa et al., 2016), and, therefore, sensitive to subsequent alteration processes. Evidence of change due to contact with the supergenic environment is strong, as it constitutes a substrate of swamps and lakes, giving rise to the formation of siderite and (Ca, Fe2 +, Fe3+) phosphates such as dufrenite, mitridatite and beraunite (Costa & Costa, 1980; Costa, 1982; Costa, 1990; Costa & Lemos, 2000; Lemos et al., 2007; Costa et al. 2018). In Bonito, these green phosphates associated with swampy events have been described (Costa et al., 2018), in the same way in other deposits such as Jandiá and Trauira (Costa, 1982). Therefore, there is no doubt that the phosphate iron crusts of Bonito were also the stage of alteration, involving the influence of swamp and probably early stages of pedogenesis, as illustrated by deep root perforations, or that these are still the effect of the past swampy environment. As stated, the mode of occurrence of wavellite in Bonito, demonstrates in fact a partial alteration, with dissolution and wavellite neoformation, in addition to goethite and hematite.
The aluminum phosphates of the alunite supergroup mentioned earlier and constituents of the phosphate ore of Bonito, are not stable in the presence of tropical weathering when near to the surface, dissolving, as can be demonstrated by the reaction below:
variscite + 2Al(OH)3 + 4H+ + HPO42- = wavellite + 2H2O
This happens when surface aqueous fluids, due to the influence of decomposing organic matter, become more acid, releasing Al(OH)3 and HPO42- complexes by partially altering the previous phosphates. The increase in the concentrations of these two chemical components in the presence of relics of variscite, for example, promote the formation of wavellite. As an example, variscite was used, but it could be simply crandallite-goyazite and/or woodhouseite-svanbergite, among others. The formation of aluminum phosphates in tropical soils is recurrent, when there is an excess of natural or anthropogenic phosphor addition, which has been known to soil science for a long time (Kittrick & Jackson, 1955; Nriagu, 1976). These transformations with respective fields of stability are shown very clearly in Figure 11, as a product of the works of Vieillard, et al. (1979), Oliveira (1980), Flicoteaux (1982), Flicoteaux & Lucas (1984), Schwab et al. (1996). This diagram shows that in fact when decreasing the pH and increasing the H3PO4 activity, crandallite and/or woodhouseite converges to, for example, augelite or else wavellite, the latter being preferred when the activity of H3PO4 is higher. According to Oliveira (1980), the preferential formation of wavellite will also depend on the concentration of H4SiO4 in the aqueous solution, as it decreases, the wavellite will form preferentially, when augelite and variscite may also precipitate.
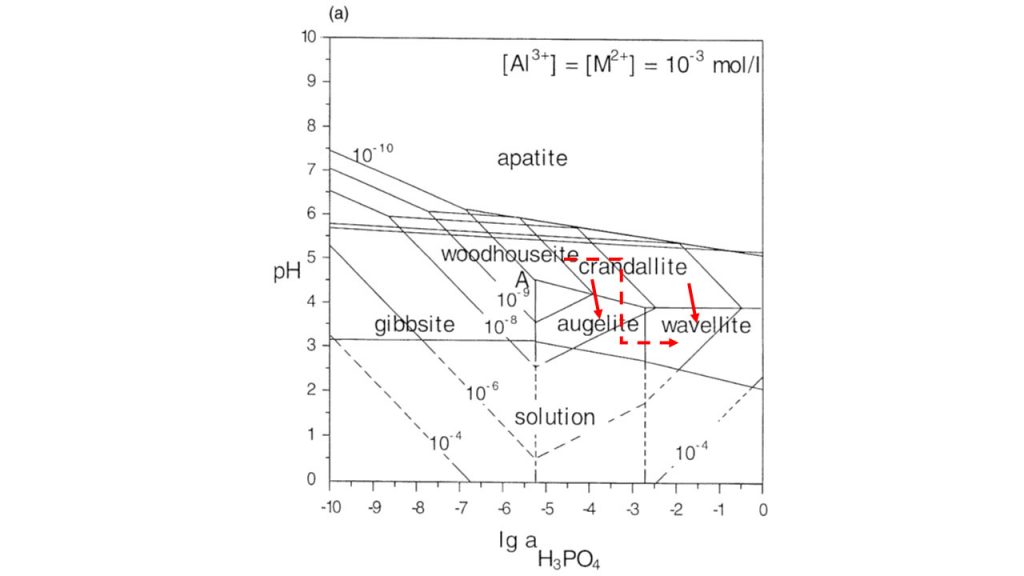
Figure 11 – Phase diagrams showing the pH and log a H3PO4 activities of various APS minerals common to laterites after Schwab et al. (1996), modified by Dill (2001). Please see field stability condition for formation of wavellite. The red arrows indicate the pathway of chemical reactions and mineral neoformation.
CONCLUSIONS
Wavellite is a typical mineral in the cavities of the aluminum phosphate iron crusts of Sapucaia, in Bonito, Pará, capping a typical mature laterite profile. It is found as well-formed, transparent and colorless millimeter crystals. The cavities are developed in yellowish white to light brown phosphate cement, which surrounds the red nodules and concretions of hematite and goethite. Bonito’s wavellite presents a diffractometric spectrum similar to the other wavellites, however, when refined, it shows to be low in fluorine, and for this reason, in addition to others, its chemical composition is not at all compatible with the theoretical wavellite rich in fluorine.
Its mode of occurrence and considering the pH – chemical activity of H3PO4 stability diagram, allow us to conclude that the Bonito wavellite is the product of an untimely near-surface chemical alteration of the aluminum phosphate iron crusts, to which they were exposed. As a consequence of local decomposition, they developed centimetric cavities and aqueous solutions rich in Al(OH)3 and (HPO4)2- complexes, which saturated favored the formation of wavellite.
Acknowledgements
The authors thank to CNPQ for continued financial support through Grants (Proc. 305015/ 2016-8); to the LAMIGA laboratories at IG/UFPA, IG/UnB and the Institut für Geowissenschaften at the University of Halle-Wittenberg, Germany, for their analytical support.
REFERENCES
Brandt, F., 1932. Ein neuer Typ von Eisen-Tonerde-Phosphat-Vorkommen (Maranhão Nordbrasilien). Chem. Erde 7, 383–425.
Capedcomme, L., 1953. Estude minéralogique des gites de phosphatés alumineux de la région de Thiés (Sénegal). Congr. Géol. Int. Comptes Rendus, XI Session, Alger, pp. 103–118.
Costa, M.L. 1979. Geologia, mineralogia, geoquímica e gênese dos fosfatos de Jandiá, Cansa-Perna e Itacupim no PA e Pirocaua e Trauíra no MA. Belém. 164p. Dissertação de Mestrado em Geociências, Núcleo de Ciências Geofísicas e Geológicas, Universidade Federal do Pará.
Costa, M.L., 1982. Petrologisch-geochemische Untersuchungen zur Genese der Bauxite und Phosphat-Laterite der Region Gurupi. (Ost-Amazonien) (Ph.D thesis) University of Erlangen, Germany, Erlangen, 190 pp.
Costa, M.L., 1990. Mineralogia, gênese e epigênese dos lateritos fosfáticos de Jandiá, na região Bragantina (NE do Pará). Geochim. Bras. 4 (1), 85–110.
Costa, M.L., Sá, J.H.S., 1980. Os fosfatos lateríticos da Amazônia Oriental: Geologia, Mineralogia, Geoquímica e Correlação com as Bauxitas da Amazônia. SBG, Congresso Brasileiro Geologia 3, pp. 1459–1472.
Costa, M.L., Costa, W.A.M., Schwab, R.G., 1980. Mineralogia das ocorrências de fosfatos lateríticos do Pará e Maranhão (Brasil). SBG, Congresso Brasileiro Geologia. Balneário de Camboriú, Anais 4, pp. 1982–1996.
Costa, M.L., Reymão, M.F.F. 1984. Senegalita, Al2(OH)3(H2O)(PO4), nos fosfatos lateríticos do Pará e Maranhão (Amazônia Oriental). Revista Brasileira de Geociências, 14(3): 170-174.
Costa M.L.; Costa W.A.M. 1987. Distribuição dos terras raras na solução sólida crandallita-goyazita de Sapucaia (Bonito-Pará). In: IV Congresso Brasileiro de Geoquímica, vol. I, p. 53-69.
Costa, M.L., Lemos, V.P. 2000. Siderita e vivianita em crostas lateríticas alteradas epigeneticamente (Padauari, Amazonas). REM, 53 (2): 101-107.
Costa J.L. 2000. Folha Castanhal (SA-23-V-C). Estado do Pará, escala 1:250.000 (Programa de Levantamentos Geológicos Básicos do Brasil/Programa Grande Carajás). Brasília, CPRM, CD-ROM.
Costa, M.L., Leite, A.S., Pöllmann, H. 2016. A laterite-hosted APS deposit in the Amazon region, Brazil: The physical-chemical regime and environment of formation. Journal of Geochemical Exploration 170 (2016) 107–124. http://dx.doi.org/10.1016/j.gexplo.2016.08.015.
Costa, M.L., Kaden, R., Pöllmann, H., Leite, A.S. 2016. Wavellita na crosta ferroaluminosa fosfática da mina de fosfatos de Bonito. BOMGEAM, 3(2) (2016): 1-1.
Costa, M.L., Valente, G.J.S.S., Queiroz, A.F.S., Santos, P.R.C. 2018. Fosfatos verdes da crosta ferro-aluminosa-fosfática da mina de fosfatos de alumínio de Bonito (Pará). BOMGEAM, 5 (3): 1-12. Doi: 10.31419/ISSN.2594-942X.v52018i3a13MLC.
Costa, W.A.M., Costa, M.L., Ferreira, M.J.C., 1991. Espectro de minerais pesados no perfil laterítico fosfático de Sapucaia (nordeste do Pará). III Simpósio de Geologia da Amazônia. Belém-Pará. Anais, pp. 512–526.
Dill, H.G., 2001. The geology of aluminum phosphates and sulphates of the alunite group minerals: a review. Earth-Sci. Rev. 53, 35–93.
Dill, H.G., Fricke, A., Henning, K.-H. 1995, The origin of Ba- and REE-bearing aluminium-phosphate-sulphate minerals from the Lohrheim kaolinitic clay deposit (Rheinisches Schiefergebirge, Germany). Applied Clay Science 10 (1995) 231-245.
Flicoteaux, R., 1982. Genese des phosphates alumineux du Senegal occidental, etapes et guides de l’alteration. Sciences Geologiques, Memoire 67, 1–249.
Flicoteaux, R., Lucas, J., 1984. Weathering of phosphate minerals. In: Nriagu, J.O., Moore, P.B. (Eds), Phosphate Minerals. Springer, Berlin, pp. 292–317.
Jambor, J.L., 1999. Nomenclature of the alunite supergroup. Canadian Mineralogist 37, 1323–1341.
Johan, Z., 1976. Sénégalite, Al2(PO4)(OH)3.H2O, un noveau mineral. Lithos, 9: 165-171.
Kaden, R., Costa, M.L., Pöllmann, H. No date. On the thermal behaviour of wavellite and the role of the fluoride content on the crystal structure and phase transition by thermal dehydration. Not published, 26 p., ill., abstract.
Kittrick, J.A., Jackson, M.L. 1955. Rate of phosphate reaction with soil minerals and Electron microscope. Observation on the reaction mechanisms. Soil Sci. Soc.Am., 19: 292-295.
Leite, A. S., 2014. Geologia, mineralogia e geoquímica dos fosfatos de Sapucaia (Bonito-PA). Dissertação de Mestrado, PPGG/IG/UFPA, Belém, Pará, 94p.
Lemos, V.P., Costa, M.L., Lemos, R.L., Faria, M.S.G., 2007. Vivianite and siderite in lateritic iron crust: an example of bioreduction. Quim. Nova, 30 (1): 36-40.
Nriagu, J.O. 1976. Phosphate-clay mineral relations in soil and sediments. Canadian Journal of Earth Sciences, 13:719-736.
Oliveira, N.P. 1980. Mineralogie und geochemie der phosphatführenden Lateriten von Itacupim und Trauira, Nordbrasilien.Thesis, Universität Erlangen-Nürnberg, Erlangen, 149 p.
Oliveira, N.P., Schwab, R.G., 1980. Itacupim: Um exemplo da influência do fósforo sobre o desenvolvimento de perfis lateríticos. Anais do XXXI Congresso Brasileiro Geologia. Santa Catarina, pp. 184–196.
Pöllmann, H., Wenda, R., Costa, M., 1987. Senegalit, ein seltenes Phosphat von Pirocaua und cansa Perna/NE Brasilien. Aufschluß 38, 303–307.
Santos, D. C. 2019. Gamaespectometria aplicada à prospecção de fosfatos lateríticos na região do Gurupi – NE do Pará e NW do Maranhão. Dissertação de mestrado, PPGABFM/FGEL/UERG, 76p.
Schwab, R.G., Costa, M.L., Oliveira, N.P., 1983. Über die Entwicklung von Bauxiten und Phosphat-Laterite der Region Gurupi (Nord-Brasilien). Zbl. Geol. Paläont. Teil 1 (3/4), 563–580.
Schwab, R.G., Herold, H., Costa, M.L., Oliveira, N.P., 1989. The formation of aluminous phosphates through lateritic weathering of rocks. In: Balasubramanian, K.S., Evangelov, V.P. (Eds.), Weathering: Its Products and Deposits 2. Theophrastus, Athens, pp. 369–386.
Schwab, R.G., Götz, C., Herold, H., Pinto de Oliveira, P., 1993. Compounds of the crandallite type: thermodynamic properties of Ca-, Sr-, Ba-, Pb-, La-, Ce- to Gd-phosphate and arsenates. N. Jb. Miner. Mh. 1993, 551–568.
Shaw, E. W. Wright W. H. and Darnell, Jas. L. JR. 1925. The Mineral Resources of Maranhão, Brazil. Economic Geology, 20: 723-728.
Slansky, M., Lallemand, A., Millot, C., 1964. La sédimentation et l’altération latéritique des fomations phosphatés des Gisements de Taoba (République de Sénégal). Bull. Serv. Carte Géol. Als. Lorr. Strasbourg 17 (4), 311–324.
Toledo, M.C.M, Oliveira, S.M.B., Costa, M.L., Passos, C.M., Almeida, H.D. 2006. Evolução do Manto de Intemperismo Laterítico Rico em Fosfatos na Ilha de Itacupim (PA) – Mineralogia, Micromorfologia e Geoquímica. Pesquisas em Geociências, 33 (2): 109 – 122.
Vieillard, P., Tardy, Y., Nahon, D. 1979. Stability fields and aluminum phosphates: paragenesis in lateritic weathering of argilaceous phosphatic sediments. Am. Mineral. 64, 626–634.
![]() 10.31419/ISSN.2594-942X.v72020i1a2MLC
10.31419/ISSN.2594-942X.v72020i1a2MLC